Professional Documents
Culture Documents
Biology Practical Activity Surface Area and Rate of Reaction
Uploaded by
Christopher OngCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Biology Practical Activity Surface Area and Rate of Reaction
Uploaded by
Christopher OngCopyright:
Available Formats
Christopher Ong 11BIOL4
Biology Practical Activity: Surface Area and Rate of Reaction
Syllabus Outcomes: 8.3.3.3.2
Perform a first-hand investigation to demonstrate the relationship between surface area and rate of reaction
Aim: To investigate how surface area affects the rate of reaction. Risk Assessment: Identify Acid splash into eye Rating Low Minimisation Wear Safety Glasses. Use low strength acid. If contact occurs, wash eyes for several minutes Do not cool immediately after heating. Hold the beaker securely. Keep the beaker away from the edge of the bench. Wear gloves. Use low strength acid. If contact occurs, wash fingers immediately.
Cut from broken beaker glass
Low
Acid splash onto fingers
Low
Equipment: - 3 antacid tablets - 1 measuring cylinder - 3 50mL beakers - 1 150mL beaker filled with 60mL HCl (Hydrochloric Acid) - 1 stopwatch - Mortar and Pestle - Safety Glasses Procedure: 1. Wear safety glasses before performing the experiment. 2. Collect the above materials and bring them to your bench. 3. Measure exactly 20mLs of HCl using a measuring cylinder. 4. Pour the HCl into a 50mL beaker. 5. Repeat steps 3 and 4 for two more beakers. 6. Unwrap all the antacid tablets from their packaging. 7. Crush one tablet into a fine powder using the mortar and pestle. 8. Break another tablet into half, leaving the remaining tablet whole. 9. At the same time, drop the whole tablet, half-broken tablet and the crushed tablet into separate beakers with 20mLs of HCl. It should look similar to Figure 1. 10.
Figure 1 Tablets dissolving in acid
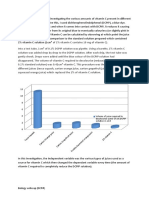
Christopher Ong 11BIOL4 11. Start the stopwatch as soon as the tablet drops into the acid. 12. Lap the stopwatch every time the tablet dissolves, stopping the timer when the third tablet has finished fizzing. 13. Record and tabulate the times at which the tablets have completely dissolved. 14. Return the equipment and clean the bench space. Results: Type of Tablet 1. Whole 2. Half-broken 3. Crushed Minutes 2.75 2.52 2.00 Time taken to dissolve Seconds 165 151 120
1 Discussion:
1. It is necessary to avoid contact between hydrochloric acid and the skin/eyes because it is corrosive, and can cause burns on contact. If it comes in contact with the eyes, it can result in blindness, depending on the strength of the acid. To prevent contact of hydrochloric acid with the eyes, safety glasses can be worn to protect from splashes. To prevent contact with the skin, gloves can be worn, or a dropper can be used to handle the liquid. To reduce the danger of the acid, a weaker or more diluted acid can be used. 2. The independent variable in this experiment is how crushed the tablet is. The dependent variable in this experiment is the time it takes to dissolve. 3. The controlled variables in the experiment are the amount of hydrochloric acid, the size of the tablet and the size of the beaker. 4. The rate of reaction can also be called the speed of reaction. The rate is how fast or slow the reaction takes place. It is a quantity measured with respect to another measured quantity. In the practical, the speed of which the tablet dissolved was compared to how crushed the tablet was. 5. From our results, we can determine that the greater the surface area, the faster the reaction time. Similarly, the lower the surface area, the slower the reaction time. The crushed tablet, which has greater surface area, dissolved 45 seconds faster than the whole tablet, which took 165 seconds to dissolve. The half-broken tablet, took 31 seconds longer than the crushed tablet, but dissolved faster than the whole tablet by 14 seconds.
Christopher Ong 11BIOL4 6. From the information given, we can see that when an antacid tablet comes in contact with an acid, it produces carbon dioxide, water and a salt. When the antacid tablet is digested in the stomach, it reacts with the acid in the stomach, producing large amounts of carbon dioxide. This excess carbon dioxide is belched out, soon after taking the antacid tablet. The antacid tablet provides digestive relief, as it helps to neutralise the acidity of the stomach. It is especially effective in reducing heartburn, as the acid has been neutralised. Improvements 1. The experiment can be made more reliable if a larger beaker with more hydrochloric acid is used. The problem encountered was that the bubbles produced by the reaction prevented some of the powdered tablet from dissolving. It was only when the bubbles popped that the powdered tablet came in contact with the acid, and this may have affected reaction times. 2. The accuracy of the experiment can be improved, if calibrated equipment is used to measure the amount of hydrochloric acid being used in the experiment. It is likely that each beaker had a slightly different amount of hydrochloric acid in them, due to human or parallax error. By weighing the contents of the beaker with acid, exactly the same amount of acid can be used for each beaker.
Conclusion: Through the experiment performed, we successfully investigated how surface area affects rate of reaction. The crushed tablet dissolved the fastest, followed by the half-broken tablet, and lastly the whole tablet, when dropped in acid. From these results, it was found that a greater surface area resulted in a faster rate of reaction. Similarly, a lower surface area resulted in a slower reaction rate.
You might also like
- Rate of Reaction Lab - ProcedureDocument7 pagesRate of Reaction Lab - ProcedureSara Alexander100% (3)
- MR Thomas ScienceDocument3 pagesMR Thomas ScienceScienceatGreenmountNo ratings yet
- The Effect of The Level of PH On The Reaction Rate of Alka Selzter Tablet001Document7 pagesThe Effect of The Level of PH On The Reaction Rate of Alka Selzter Tablet001bunnyismeNo ratings yet
- 1 - Difussion and OsmosisDocument11 pages1 - Difussion and OsmosisLisa Jin100% (1)
- Chloride SalinityDocument9 pagesChloride SalinityBoby ThomasNo ratings yet
- Chemistry Lab Report 1Document18 pagesChemistry Lab Report 1Daniel Duane LimNo ratings yet
- Lab On Transpiration BioDocument8 pagesLab On Transpiration BioNigg100% (1)
- Communication Studies Exposition IADocument3 pagesCommunication Studies Exposition IADana SpringerNo ratings yet
- Biology November ReportDocument3 pagesBiology November ReportIndrani GoswamiNo ratings yet
- Nail rusting experimentDocument2 pagesNail rusting experimentemilieNo ratings yet
- Chemistry Investigatory ProjectDocument23 pagesChemistry Investigatory ProjectDipangshuman ChoudhuryNo ratings yet
- Partition Coefficient DeterminationDocument4 pagesPartition Coefficient DeterminationMostafa HamawandyNo ratings yet
- 5 Transpiration LabDocument3 pages5 Transpiration LabLauren Delgado100% (1)
- Determination of Sugar As GlucoseDocument4 pagesDetermination of Sugar As GlucoseIpsita ChakravartyNo ratings yet
- Amylase Enzyme and Temperature LabDocument4 pagesAmylase Enzyme and Temperature LabJames DaurayNo ratings yet
- IA Enthalpy ChangeDocument6 pagesIA Enthalpy ChangeAldo HamkaNo ratings yet
- Bio ReportDocument8 pagesBio ReportTharshini_Indr_6713No ratings yet
- Acids Bases SaltsDocument74 pagesAcids Bases SaltshaloNo ratings yet
- Rates of ReactionDocument77 pagesRates of ReactionFatema KhatunNo ratings yet
- Oecd 202 211 Daphnia MagnaDocument0 pagesOecd 202 211 Daphnia MagnaHeidita SanchezNo ratings yet
- Dicot Root AnatomyDocument1 pageDicot Root AnatomyMuhammedHaneefNo ratings yet
- Envi Ia 2018Document43 pagesEnvi Ia 2018Nkosi JupiterNo ratings yet
- Introduction to Analytical Methods for Environmental Chemical AnalysesDocument4 pagesIntroduction to Analytical Methods for Environmental Chemical Analysesmasitule nkombisaNo ratings yet
- Experiment 5Document32 pagesExperiment 5Saniha Aysha AjithNo ratings yet
- Ecological Study Report F6Document89 pagesEcological Study Report F6dreamz28No ratings yet
- Rate of Reaction ReportDocument9 pagesRate of Reaction ReportKevin ReviroNo ratings yet
- Lab Photosynthesis InquiryDocument6 pagesLab Photosynthesis Inquirycorygunther100% (1)
- Enzymatic Browning and Its Prevention-American Chemical Society (1995)Document340 pagesEnzymatic Browning and Its Prevention-American Chemical Society (1995)danielguerinNo ratings yet
- DCPIP Write UpDocument2 pagesDCPIP Write UpHashim Chishty0% (1)
- Water TreatmentDocument19 pagesWater TreatmentAnonymous 8ooQmMoNs1No ratings yet
- Neutralization of Acid and BaseDocument18 pagesNeutralization of Acid and BaseMara PhotxNo ratings yet
- Determining The Amount of "Antacid" in An Antacid TabletDocument11 pagesDetermining The Amount of "Antacid" in An Antacid TabletYsabel Del FierroNo ratings yet
- Alkenes and AlkanesDocument5 pagesAlkenes and AlkanesLisWeiNo ratings yet
- Regulation of climacteric and non-climacteric fruit ripening pathways in melonDocument7 pagesRegulation of climacteric and non-climacteric fruit ripening pathways in melonsandraosouzaNo ratings yet
- CHM2201 - LECTURE 2 METHODSDocument31 pagesCHM2201 - LECTURE 2 METHODSAcidri Abdulkarim100% (1)
- Joshua Haholongan - Science Rate of Reaction ReportDocument13 pagesJoshua Haholongan - Science Rate of Reaction ReportJoshua HaholonganNo ratings yet
- Standardization of Volumetric GlasswareDocument8 pagesStandardization of Volumetric GlasswareJIEHASMARTNo ratings yet
- SCIENTIFIC PAPER (Chemistry Lab) - Determination of DensitiesDocument4 pagesSCIENTIFIC PAPER (Chemistry Lab) - Determination of DensitiesMi Rivera75% (4)
- Titration Lab Report MYP ChemistryDocument14 pagesTitration Lab Report MYP ChemistryHardik SinghiNo ratings yet
- Ichem LabDocument4 pagesIchem LabDiana BunaganNo ratings yet
- AP Lab 9 TranspirationDocument7 pagesAP Lab 9 TranspirationElioth GomezNo ratings yet
- Biology Catalase Experiment DesignDocument6 pagesBiology Catalase Experiment DesignNimisha SharmaNo ratings yet
- Experiment 4 Electrochem CMT555Document10 pagesExperiment 4 Electrochem CMT555Amar Safwan100% (1)
- Enzyme ConcentrationDocument16 pagesEnzyme ConcentrationSya Subi100% (1)
- Browning ProcessDocument40 pagesBrowning ProcessRendy AndikaNo ratings yet
- Alka Seltzer Lab ReportDocument4 pagesAlka Seltzer Lab ReportJeffery Osvold100% (2)
- Chromatography 05Document5 pagesChromatography 05Snow DropNo ratings yet
- Com Studies IaDocument10 pagesCom Studies IakrisNo ratings yet
- Titus John - Enthalpy Prac ReportDocument12 pagesTitus John - Enthalpy Prac Reportapi-295071132No ratings yet
- Determination of Oxygen Dissolved in Water by WinklerDocument3 pagesDetermination of Oxygen Dissolved in Water by WinklerSazzan NpnNo ratings yet
- Chemistry PDDocument2 pagesChemistry PDTammara Wallace100% (1)
- Calorimetry (Formal)Document17 pagesCalorimetry (Formal)Bettinamae Ordiales De Mesa0% (1)
- 124 Melting Point2Document15 pages124 Melting Point2bluestardiverNo ratings yet
- PolymersDocument8 pagesPolymersLauren LloydNo ratings yet
- Principles of Chemical EquilibriumDocument17 pagesPrinciples of Chemical EquilibriumkaditasookdeoNo ratings yet
- Effect of pH on Enzymatic Browning of ApplesDocument7 pagesEffect of pH on Enzymatic Browning of ApplesAlark SharmaNo ratings yet
- Properties of Water at Home LabDocument5 pagesProperties of Water at Home Labkim_peplerNo ratings yet
- Title of The Lab: Enzyme Lab Research Question: What Is The Effect of Decreasing Concentration of HDocument3 pagesTitle of The Lab: Enzyme Lab Research Question: What Is The Effect of Decreasing Concentration of HDevanshi SinghalNo ratings yet
- Introductory Titrimetric and Gravimetric Analysis: The Commonwealth and International Library: Chemistry DivisionFrom EverandIntroductory Titrimetric and Gravimetric Analysis: The Commonwealth and International Library: Chemistry DivisionNo ratings yet
- New Chemistry CurriculumDocument111 pagesNew Chemistry Curriculumkiya0175% (4)
- Hydrogen Storage Using LOHC SystemDocument95 pagesHydrogen Storage Using LOHC SystemMuhammad AbdullahNo ratings yet
- (Patel V., (Ed.) (2012) ) Chemical Kinetics - INTEC PDFDocument354 pages(Patel V., (Ed.) (2012) ) Chemical Kinetics - INTEC PDFGeorgeisNo ratings yet
- MCAT Organic ChemistryDocument7 pagesMCAT Organic ChemistryjoNo ratings yet
- (SSC) Consumer Chemistry9 Q1 M6 W6Document24 pages(SSC) Consumer Chemistry9 Q1 M6 W6.No ratings yet
- T.Y.B.Sc. (CHEMISTRY) Revised Syllabus From June 2010 (Semester System) Structure of The Syllabus First Term (Semester Iii) Compulsory CoursesDocument71 pagesT.Y.B.Sc. (CHEMISTRY) Revised Syllabus From June 2010 (Semester System) Structure of The Syllabus First Term (Semester Iii) Compulsory CoursesHardi AhmedNo ratings yet
- When Gold Is Not NobleDocument13 pagesWhen Gold Is Not NobleSumit KumarNo ratings yet
- Lecture 22Document16 pagesLecture 22imania shaumiNo ratings yet
- Calculations of An 031012 MBPDocument411 pagesCalculations of An 031012 MBPLeonardo LugoNo ratings yet
- ChemistryDocument3 pagesChemistryRohak KanojiaNo ratings yet
- HOTS OrganicDocument3 pagesHOTS Organicsohil khattarNo ratings yet
- Vol 43 - 3 0004Document112 pagesVol 43 - 3 0004karamniaNo ratings yet
- Part - I: Objective Questions: Section (A) : Unimolecular Nucleophilic Substitution Reactions of Alkyl Halides (S 1)Document24 pagesPart - I: Objective Questions: Section (A) : Unimolecular Nucleophilic Substitution Reactions of Alkyl Halides (S 1)Deeptam BharNo ratings yet
- WholeDocument397 pagesWholedelbot01No ratings yet
- Synthetic and Systems Biotechnology: Lin Wang, Satyakam Dash, Chiam Yu NG, Costas D. MaranasDocument10 pagesSynthetic and Systems Biotechnology: Lin Wang, Satyakam Dash, Chiam Yu NG, Costas D. MaranasDaniel ChamorroNo ratings yet
- Aerobic Cellular Respiration Digital Lab PosterDocument1 pageAerobic Cellular Respiration Digital Lab Posterapi-309712203No ratings yet
- Industrial Chemistry MCQDocument69 pagesIndustrial Chemistry MCQSatvik BeheraNo ratings yet
- Mathematics Arithmetic and Number Sense Algebra Geometry: (Answer Many Word Problems As Possible)Document5 pagesMathematics Arithmetic and Number Sense Algebra Geometry: (Answer Many Word Problems As Possible)Claire LouisNo ratings yet
- Determine Iron Content Using Permanganometric TitrationDocument13 pagesDetermine Iron Content Using Permanganometric TitrationNahzim RahmatNo ratings yet
- Production of Conventional Liquid Fuels From SugarsDocument14 pagesProduction of Conventional Liquid Fuels From SugarsNuc LeusNo ratings yet
- Dithiophosphonate Coordination ChemistryDocument48 pagesDithiophosphonate Coordination ChemistryLuis Fancisco Alcaraz BlancasNo ratings yet
- Bioprocess Technology Unit IDocument110 pagesBioprocess Technology Unit Ipremkumar164No ratings yet
- Kidde Fenwal - FM-200Document8 pagesKidde Fenwal - FM-200riesgoquimco drummondltdNo ratings yet
- 2019 Usnco Exam Part III PDFDocument18 pages2019 Usnco Exam Part III PDFFernando RiosNo ratings yet
- Organic Chemisty - Reaction MechanismsDocument67 pagesOrganic Chemisty - Reaction MechanismsKamrul Alam MasumNo ratings yet
- 6 ReportDocument31 pages6 ReportAbhi Butani100% (1)
- OCR AS Chemistry Ebook - OXBOXDocument136 pagesOCR AS Chemistry Ebook - OXBOXBob SmithNo ratings yet
- Synthesis and Characterization of Tungsten Carbonyl Complexes Containing ThioamidesDocument7 pagesSynthesis and Characterization of Tungsten Carbonyl Complexes Containing ThioamidesRasel MahfujNo ratings yet
- Sat450 EngDocument2 pagesSat450 EngMahamad MahmndarNo ratings yet