Professional Documents
Culture Documents
Work Done by An Expanding Gas
Uploaded by
streetrat133Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Work Done by An Expanding Gas
Uploaded by
streetrat133Copyright:
Available Formats
Work Done by an Expanding Gas
Learning Goal: To derive the expression for the work done by an expanding gas, to understand how it follows from the expression for mechanical work. , and
Especially from the historically important perspective of making engines to convert heat energy into work, the work in thermodynamics is defined as the work done by the system on the exterior world, and not vice versa as is done in the rest of classical mechanics. In classical mechanics, one always considers the work done on a system by the outside world. Rarely does one think about the work done by the system. Suppose you push a large block with a certain force of magnitude over some distance. You have done work on the block; hence the energy of the block should increase. According to Newton's 3rd law, the block exerts the same magnitude of force , but in the opposite direction (i.e., directed back at you). Hence, the work done by the block (on you) is negative, since the direction of motion opposes the direction of the force. In summary, you have to be careful about the sign of the work: the same situation gives opposite signs of the work depending on whether our perspective is classical mechanics or thermodynamics. In thermodynamics, one often deals with liquids and gases that exert forces on their containers (i.e., the fluids exert pressure over an area). If the container changes volume, then this force acts through a distance and hence does work. For a steam engine, the example pictured here, the "container" is a cylinder whose volume changes as the piston slides in or out. Suppose a gas is confined within the cylinder. The pressure of the gas is , and the area of the cylinder is . Consider the work done as the gas expands, pushing the piston to the right. Call the infinitesimal distance the piston moves .
A. What force does the gas exert on the piston? (Note that the positive x axis is to the right in the figure.) Express the force in terms of , = p*A B. If the piston moves a distance , what is , the work done by the gas? , and any constants,
Express the work done by the gas in terms of given quantities. = p*A*dx C. What is , the increase in volume of the gas? and other given quantities.
Express the differential increase in terms of = A*dx
D.
Now find the work done by the gas in terms of the thermodynamic variables. Express the differential work pressure , temperature = p*dV in terms of thermodynamic variables such as the gas's , and its change in volume .
, volume
E. Suppose that the gas expands from done by the gas? Express the work in terms of = p_0*(V_1-V_0) F. ,
to
at constant pressure
. How much work
is
, and
Is the work you just computed positive or negative? positive
negative G. Assume now that the diameter of the piston is reduced by a factor of 2. What is the amount of work done by a gas of pressure , in expanding from , to ?
Express your answer in terms of
, and simple numerical factors.
= p_0*(V_1-V_0) [ Print ]
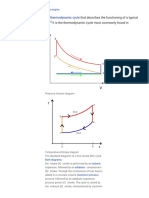
Work Integral in the pV Plane
The diagram shows the pressure and volume of an ideal gas during one cycle of an engine. As the gas proceeds from state 1 to state 2, it is heated at constant pressure. It is then cooled at constant volume, until it reaches state 3. The gas is then cooled at constant pressure to state 4. Finally, the gas is heated at constant volume until it returns to state 1. A. Find , the work done by the gas as it expands from state 1 to state 2. Express the work done in terms of and .
= 9*p_0*V_0 B. Find , the work done by the gas as it cools from state 2 to state 3. and .
Express your answer in terms of = 0
C. Find
, the work done by the gas as it is compressed from state 3 to state 4. and .
Express your answer in terms of = -3*p_0*V_0 D. Find
, the work done by the gas as it is heated from state 4 to state 1. and .
Express your answer in terms of = 0 E. What is
, the total work done by the gas during one cycle? and .
Express your answer in terms of = 6*p_0*V_0
F. When the gas is in state 1, its temperature is in state 3. (Remember, this is an ideal gas.) Express in terms of .
. Find the temperature
of the gas when it is
= (4/3)*T_1 [ Print ] [ Back to Problem View ]
Various Gas Expansions: pV Plots and Work
An ideal monatomic gas is contained in a cylinder with a movable piston so that the gas can do work on the outside world, and heat can be added or removed as necessary. The figure
shows various paths that the gas might take in expanding from an initial state whose pressure, volume, and temperature are respectively. The gas expands to a state with final volume , , and
. For some answers it will be convenient
to generalize your results by using the variable volumes (equal to 4 for the expansions shown in the figure.)
, which is the ratio of final to initial
The figure shows several possible paths of the system in the pV plane. Although there are an infinite number of paths possible, several of those shown are special because one of their state variables remains constant during the expansion. These have the following names: Adiabiatic: No heat is added or removed during the expansion. Isobaric: The pressure remains constant during the expansion. Isothermal: The temperature remains constant during the expansion.
A. Which of the curves in the figure represents an isobaric process? A B C D B. What happens to the temperature of the gas during an isobaric expansion? Temperature increases. Temperature remains constant. Temperature decreases. C. Which of the curves in the figure represents an isothermal process? A B C D D. Graphically, the work along any path in the pV plot ____________. is the area to the left of the curve from is the area under the curve from to to
requires knowledge of the temperature E. Calculate Express , the work done by the gas as it expands along path A from in terms of , , and . to .
= p_0* V_0 * (R_v - 1) F. Calculate the work Express in terms of done by the gas during the isothermal expansion. , , and .
= p_0*V_0*ln(R_v) G. Which of the curves shown represents an adiabatic expansion?
D [ Print ]
Fast vs. Slow Tire Pumping
Imagine the following design for a simple tire pump. The pump is filled with a volume atmospheric pressure and ambient temperature of air at
. When you push the pump handle, the air is
compressed to a new (smaller) volume
, raising its pressure. A valve is then opened, allowing air to
flow from the pump into the tire until the remaining air in the pump reaches the pressure of the air in the tire, . In this problem, you will consider whether you can get more air into the tire per pump cycle by pushing the pump handle quickly or slowly. We will make the following simplifying assumptions: The pressure of the air in the tire, , does not change significantly as air flows into the tire from the pump. The temperature of the air in the pump does not change significantly while air is flowing into the tire (i.e., this is an isothermal process). The air is mainly composed of diatomic molecules with .
A. First imagine that you push the pump handle quickly, so that the compression of air in the pump occurs adiabatically. Find the absolute temperature rapid compression from volume Express your answer in terms of T_a*(V_i/V_f)^(gamma-1) or = T_a*(V_i/V_f)^(0.40) B. Once the tire and pump pressures have equilibrated at , what fraction particles initially in the pump will have ended up in the tire? Express the fraction in terms of , should not appear in your answer. 1-(p_t/p_a)*(V_f/V_i)^gamma or = 1-(p_t/p_a)*(V_f/V_i)^(1.40) C. Now imagine that you push the pump handle slowly, so that the compression of air in the pump occurs isothermally. Find the absolute temperature assuming an ambient temperature of = T_a D. Once the tire and pump pressures have equilibrated at , what fraction particles initially in the pump will have ended up in the tire? Express the fraction in terms of , should not appear in your answer. = 1-(p_t/p_a)*(V_f/V_i) E. Assume that and . Which method, fast pumping (adiabatic process) or slow pumping (isothermal process) will put a larger amount of air into the tire per pump cycle? , , of the gas . of the air inside the pump , , of the gas to volume , , of the air inside the pump after a .
, assuming an ambient temperature of , and
, and . The temperatures
and
, and . The temperatures
and
fast pumping slow pumping
Carnot Cycle
After Count Rumford (Benjamin Thompson) and James Prescott Joule had shown the equivalence of mechanical energy and heat, it was natural that engineers believed it possible to make a "heat engine" (e.g., a steam engine) that would convert heat completely into mechanical energy. Sadi Carnot considered a hypothetical piston engine that contained moles of an ideal gas, showing first that it was reversible, and most importantly that--regardless of the specific heat of the gas--it had limited efficiency, defined as , where is the net work done by the engine and is the quantity
of heat put into the engine at a (high) temperature necessarily put an amount of heat
. Furthermore, he showed that the engine must .
back into a heat reservoir at a lower temperature
The cycle associated with a Carnot engine is known as a Carnot cycle. A pV plot of the Carnot cycle is shown in the figure. The working gas first expands isothermally from state A to state B, absorbing heat from a reservoir at
temperature . The gas then expands adiabatically until it reaches a temperature , in state C. The gas is compressed isothermally to state D, giving off heat . Finally, the gas is adiabatically compressed to state A, its original state. A. Indicate whether the following statements are true or false: 1. For the gas to do positive work, the cycle must be traversed in a clockwise manner. 2. Positive heat is added to the gas as it proceeds from state C to state D. 3. The net work done by the gas is proportional to the area inside the closed curve. 4. The heat transferred as the gas proceeds from state B to state C is greater than the heat transferred as the gas proceeds from state D to state A.
Use t for true or f for false. Separate your answers with commas.
t , f , t , f B. Find the total work done by the gas after it completes a single Carnot cycle. , , , and .
Express the work in terms of any or all of the quantities Q_h-Q_c or = Q_h*(1-T_c/T_h)
C. Suppose there are respectively, state B.
moles of the ideal gas, and the volumes of the gas in states A and B are, . Find , the heat absorbed by the gas as it expands from state A to
and
Express the heat absorbed by the gas in terms of reservoir , and the gas constant .
, the termperature of the hot
= n*R*T_h*ln(V_B/V_A) D. The volume of the gas in state C is , and its volume in state D is . Find , the magnitude of the heat that flows out of the gas as it proceeds from state C to state D. Express your answer in terms of and . , , , (the temperature of the cold reservoir),
= n*R*T_c*ln(V_C/V_D) E. Now, by considering the adiabatic processes (from B to C and from D to A), find the ratio in terms of = V_B/V_A F. Using your expressions for find a simplified expression for and (found in Parts C and D), and your result from Part E, . and .
No volume variables should appear in your expression, nor should any constants (e.g., or ). = T_c/T_h G. The efficiency of any engine is, by definition, . Carnot proved that no engine can have an efficiency greater than that of a Carnot engine. Find the efficiency of a Carnot engine. Express the efficiency in terms of and .
= 1-T_c/T_h [ Print ]
Isothermal Engine
Consider a thermal engine filled with cyclic transformation, as shown. molecules of a monatomic ideal gas. The gas undergoes a
Processes AB and CD are isothermal (constant temperature), and processes BC and DA are isochoric (constant volume). The quantities has pressure given. , , , and are defined in the figure. For example, when the gas is in state A, it ; when the gas is in state B, it has volume , but the pressure is not
and volume
Work done by the gas A. Find , the work done by the gas during process AB. , , , and/or .
Express the work in terms of = p_2*V_1*ln(V_2/V_1) B. Find
, the work done by the gas during process BC. , , , and/or .
Express your answer in terms of = 0 C. Find
, the work done by the gas during process CD. , , , and/or .
Express your answer in terms of = -p_1*V_2*ln(V_2/V_1) D. Find
, the work done by the gas during process DA. , , , and/or .
Express your answer in terms of
= 0 The internal energy Now, you will investigate the change in the gas's internal energy during each of the four segments of its cycle. You may find it helpful to recall that, for a monatomic ideal gas, the heat capacity of the gas at constant volume. A. What is the change in internal energy during the process AB? , , , and/or . , where is
Express the change in internal energy in terms of = 0 B. What is the change in internal energy
during the process BC? , , , and/or .
Express the change in internal energy in terms of = (3/2)*(p_1*V_2-p_2*V_1) C. What is the change in internal energy Express your answer in terms of = 0 D. What is the change in internal energy Express your answer in terms of , , , ,
during the process CD? , and/or .
during the process DA? , and/or .
= -(3/2)*(p_1*V_2-p_2*V_1) E. What is the total change in the gas's internal energy after it has completed a cycle?
= 0 Heat Finally, consider the heat absorbed by the gas during its cycle. Define to be heat absorbed by the gas in process AB, etc. If a process is exothermic (i.e., heat is given off by the gas during the process), then the quantity of heat absorbed will be negative. A. Indicate the processes in this cycle during which heat is flowing into the gas. That is, indicate the segments where is positive.
DA only CD and DA DA and AB AB and BC [ Print ]
Heat Pumps and Refrigerators
Learning Goal: To understand that a heat engine run backward is a heat pump that can be used as a refrigerator. By now you should be familiar with heat engines--devices, theoretical or actual, designed to convert heat into work. You should understand the following: 1. 2. Heat engines must be cyclical; that is, they must return to their original state some time after having absorbed some heat and done some work). Heat engines cannot convert heat into work without generating some waste heat in the process.
The second characteristic is a rigorous result even for a perfect engine and follows from thermodynamics. A perfect heat engine is reversible, another result of the laws of thermodynamics. If a heat engine is run backward (i.e., with every input and output reversed), it becomes a heat pump
(as pictured schematically must be put into a heat pump, and it then pumps heat from a colder temperature
). Work to a hotter
temperature , that is, against the usual direction of heat flow (which explains why it is called a "heat pump"). The big surprise is that the laws of thermodynamics seem to allow more than 100% efficiency: The heat coming out the hot side of a heat pump or the heat going in to the cold side of a refrigerator is more than the work put in; in fact it can be many times as large. For this reason, the ratio of the heat to the work in heat pumps and refrigerators is called the coefficient of performance. In a refrigerator, this is the ratio of heat removed from the cold side . In a heat pump the coefficient of performance is the ratio of heat exiting the hot side in: . A. What is the relationship of Express = -W in terms of to the usual thermodynamic definition of work and other quantities given in the introduction. ? to the work put to work put in:
B. Find Express
, the heat pumped out by the ideal heat pump. in terms of and .
= W_in + Q_c C. A heat pump is used to heat a house in winter; the inside radiators are at and the outside , its
heat exchanger is at . If it is a perfect (e.g., Carnot cycle) heat pump, what is coefficient of performance? Give your answer in terms of = T_h/(T_h - T_c) and .
D. The heat pump is designed to move heat. This is only possible if certain relationships between the heats and temperatures at the hot and cold sides hold true. Indicate the statement that must apply for the heat pump to work. and and and and . . . . , and
E. Assume that you heat your home with a heat pump whose heat exchanger is at
which maintains the baseboard radiators at . If it would cost $1000 to heat the house for one winter with ideal electric heaters (which have a coefficient of performance of 1), how much would it cost if the actual coefficient of performance of the heat pump were 75% of that allowed by thermodynamics? Express the cost in dollars.
Cost = 187.5 dollars [ Print ]
You might also like
- Historical Commentary On HBO's Chernobyl - Annotated Episode 5 ScriptDocument92 pagesHistorical Commentary On HBO's Chernobyl - Annotated Episode 5 ScriptMichael Long100% (1)
- Package CMPRSK': R Topics DocumentedDocument13 pagesPackage CMPRSK': R Topics DocumentedNavnath GodseNo ratings yet
- Choosing the Right Dampener Size to Reduce CostsDocument1 pageChoosing the Right Dampener Size to Reduce CostsnbsmaniannNo ratings yet
- Toro ECX TimerDocument31 pagesToro ECX TimerMichael Chambers0% (1)
- 10.4 Isothermal and Adiabatic ChangesDocument15 pages10.4 Isothermal and Adiabatic ChangesYuvaneshwaran SrinivasanNo ratings yet
- Optis - Ow - LM - Ug - 2014 SP1 PDFDocument251 pagesOptis - Ow - LM - Ug - 2014 SP1 PDFRaghavNo ratings yet
- Working For OilDocument435 pagesWorking For OilSyamsuddin MansurNo ratings yet
- Correlating Equations For Laminar and Turbulent Free ConveDocument7 pagesCorrelating Equations For Laminar and Turbulent Free Convearman_1287No ratings yet
- The Totally Unauthorized Obamacare Joke Book: NEW 'We've Been Grubered' EditionFrom EverandThe Totally Unauthorized Obamacare Joke Book: NEW 'We've Been Grubered' EditionNo ratings yet
- MOBIL OIL CANADA HISTORY OF EXCELLENCEDocument20 pagesMOBIL OIL CANADA HISTORY OF EXCELLENCESan BrigitteNo ratings yet
- Quiksilver Rossignol binding manual 2007-08Document48 pagesQuiksilver Rossignol binding manual 2007-08Diego AlcoNo ratings yet
- H3 ManualDocument4 pagesH3 Manualmegameca007No ratings yet
- PC1431 MasteringPhysics Assignment 8Document15 pagesPC1431 MasteringPhysics Assignment 8stpmoment44% (9)
- 4c Lab3 Heat EnginesDocument4 pages4c Lab3 Heat EnginesAiza AliNo ratings yet
- First Law of ThermodynamicsDocument22 pagesFirst Law of Thermodynamicsavi taylorNo ratings yet
- Thermodynamics: U Will BeDocument12 pagesThermodynamics: U Will BeJimNo ratings yet
- 20171030161017SFG 3023 Chapter 5Document51 pages20171030161017SFG 3023 Chapter 5Nabilah SyahirahNo ratings yet
- Carnot Heat EngineDocument10 pagesCarnot Heat EngineSheraz AliNo ratings yet
- Simple Finite Heat Release ModelDocument5 pagesSimple Finite Heat Release Modelh577680546No ratings yet
- The Energy and The Work of EngineDocument4 pagesThe Energy and The Work of EngineinventionjournalsNo ratings yet
- Gas Laws and Heat EngineDocument5 pagesGas Laws and Heat EngineHalimahNo ratings yet
- Cyclic Process Second Law EnginesDocument18 pagesCyclic Process Second Law EnginesM Khaidiz RafiNo ratings yet
- Dr. Ranojit Kumar Dutta (DRD)Document27 pagesDr. Ranojit Kumar Dutta (DRD)KRZ. Arpon Root HackerNo ratings yet
- Physics Thermodynamics ChapterDocument39 pagesPhysics Thermodynamics ChapterMuhammad SolehinNo ratings yet
- Heat Engine EfficiencyDocument8 pagesHeat Engine EfficiencyleisllyNo ratings yet
- Section 14 Dash 6Document2 pagesSection 14 Dash 6arifsheikh13dNo ratings yet
- Rudolf Diesel, A German Inventor, Patented His Internal Combustion Engine, WhichDocument6 pagesRudolf Diesel, A German Inventor, Patented His Internal Combustion Engine, WhichAsraNo ratings yet
- AREN 2110: Heat Engine ExperimentDocument8 pagesAREN 2110: Heat Engine ExperimentRafa ElaNo ratings yet
- Labsheet SKKC 2721 20162017 - 02Document32 pagesLabsheet SKKC 2721 20162017 - 02HoongNo ratings yet
- Second Law of Thermodynamics ExplainedDocument13 pagesSecond Law of Thermodynamics ExplainedSudip NeupaneNo ratings yet
- The Thermodynamics of The Internal Combustion EngineDocument25 pagesThe Thermodynamics of The Internal Combustion EnginerammanyalaNo ratings yet
- Physics - Engineering PC 1431 - Experiment P2 Heat EngineDocument6 pagesPhysics - Engineering PC 1431 - Experiment P2 Heat EnginelcblscNo ratings yet
- Cylinder Pressure Model for Spark-Ignition EngineDocument6 pagesCylinder Pressure Model for Spark-Ignition EngineShubham DeshmukhNo ratings yet
- Unit - I Concept of Compressible Flow - Sae1301Document172 pagesUnit - I Concept of Compressible Flow - Sae1301Divy KaravdiyaNo ratings yet
- P 303 AnsDocument4 pagesP 303 AnsDiptoNo ratings yet
- ME120 Project ReportDocument8 pagesME120 Project Reportaliosmans100% (2)
- Applications of TDsDocument15 pagesApplications of TDsReddyvari VenugopalNo ratings yet
- Reciprocating Compressor GuideDocument15 pagesReciprocating Compressor GuideShashank Muduli33% (3)
- Part I: Basic Concepts of Thermodynamics: - Heat and Work - Kinetic Theory of GasesDocument34 pagesPart I: Basic Concepts of Thermodynamics: - Heat and Work - Kinetic Theory of GasesAngel sitorusNo ratings yet
- P2 - Heat EngineDocument4 pagesP2 - Heat EngineSre VinodNo ratings yet
- Hydraulic Accumulator EquationsDocument7 pagesHydraulic Accumulator Equationsrsproserv0% (1)
- TALLER 3 - 2do CorteDocument9 pagesTALLER 3 - 2do Corteeylen OviedoNo ratings yet
- Heat Engines 7Document8 pagesHeat Engines 7member1000No ratings yet
- The History and Processes of the Brayton Cycle Gas TurbineDocument24 pagesThe History and Processes of the Brayton Cycle Gas TurbineShaheizy SaiNo ratings yet
- IsentropicDocument5 pagesIsentropicBane BarrionNo ratings yet
- Typical Examples of Irreversible ProcessesDocument8 pagesTypical Examples of Irreversible ProcessesadminchemNo ratings yet
- Air Standard Engine: Exhaust ProcessDocument43 pagesAir Standard Engine: Exhaust ProcessMukesh BohraNo ratings yet
- MEEG 346 Thermal Laboratory: Experiment #4: The Four-Stroke Combustion EngineDocument9 pagesMEEG 346 Thermal Laboratory: Experiment #4: The Four-Stroke Combustion EnginepsunmoorthyNo ratings yet
- Otto CycleDocument7 pagesOtto CycleklashincoviskyNo ratings yet
- AP Problems Database UhrichDocument18 pagesAP Problems Database UhrichMagesh KumarNo ratings yet
- ME6404 UwDocument92 pagesME6404 UwAnonymous Zx7EG1PaNo ratings yet
- Ic Engine Cycles 1Document85 pagesIc Engine Cycles 1jhpandiNo ratings yet
- Effect of Forced Convection on Cylinder Heat TransferDocument13 pagesEffect of Forced Convection on Cylinder Heat TransferDilshad S FaisalNo ratings yet
- Mehran University of Engineering and Technology SZAB Campus Khairpur Mir'sDocument64 pagesMehran University of Engineering and Technology SZAB Campus Khairpur Mir'sMarcusHuynh88No ratings yet
- Introduce The Function of Compressor Amd PumpDocument3 pagesIntroduce The Function of Compressor Amd PumpDhana KumaranNo ratings yet
- Entropy and The Second Law of ThermodynamicsDocument5 pagesEntropy and The Second Law of Thermodynamicsrizal123No ratings yet
- Otto Cycle - WikipediaDocument13 pagesOtto Cycle - WikipediaMichaelle Angela ArnedoNo ratings yet
- Ch19 ISMDocument84 pagesCh19 ISMJessamine KurniaNo ratings yet
- Med HX Lecture Student Handout Fall 2015Document3 pagesMed HX Lecture Student Handout Fall 2015streetrat133No ratings yet
- Temple University - Department of Chemistry CHE 3302 - Spring 2014Document1 pageTemple University - Department of Chemistry CHE 3302 - Spring 2014streetrat133No ratings yet
- 83109Document7 pages83109streetrat133No ratings yet
- Pre-Committee Interview Questions: Temple UniversityDocument2 pagesPre-Committee Interview Questions: Temple Universitystreetrat133No ratings yet
- 2043 Exam1reviewDocument1 page2043 Exam1reviewstreetrat133No ratings yet
- Phys Help2Document5 pagesPhys Help2streetrat133No ratings yet
- Answers To Practice Sheet On BONESDocument4 pagesAnswers To Practice Sheet On BONESstreetrat133No ratings yet
- Ketone Reduction Proc SP13Document1 pageKetone Reduction Proc SP13streetrat133No ratings yet
- Solutions: TankersDocument7 pagesSolutions: TankersKirtishbose ChowdhuryNo ratings yet
- Air Compressor (Proposal)Document10 pagesAir Compressor (Proposal)Conte DiazNo ratings yet
- Syllabus ME377 1445 1stDocument1 pageSyllabus ME377 1445 1stMossab QNo ratings yet
- Mirzapur Cadet College: Time: 2hours 35min. Full Marks: 50 Answer Any Five Set of Questions in The FollowingDocument4 pagesMirzapur Cadet College: Time: 2hours 35min. Full Marks: 50 Answer Any Five Set of Questions in The Followingmuktadir hosenNo ratings yet
- Kami Export - Abbas Kamoona - Caie-Igcse-Chemistry-0620-Theory-V10Document29 pagesKami Export - Abbas Kamoona - Caie-Igcse-Chemistry-0620-Theory-V10Abbas KamoonaNo ratings yet
- Catalyst Attrition in Ebullated-Bed Hydrotreator Operations: Ezra K.T. Kam, Fatma Jasam, Mohammad Al-MashanDocument12 pagesCatalyst Attrition in Ebullated-Bed Hydrotreator Operations: Ezra K.T. Kam, Fatma Jasam, Mohammad Al-MashanLindsey BondNo ratings yet
- Absorption Final ReportDocument8 pagesAbsorption Final Reporthamza A.laftaNo ratings yet
- Request For Proposal (RFP) For Establishing Isrosene Production Plant (Ipp)Document90 pagesRequest For Proposal (RFP) For Establishing Isrosene Production Plant (Ipp)vivek dakavarapuNo ratings yet
- 770-070 FGA2 Rev 2 March 2005 Instruction Manual PDFDocument146 pages770-070 FGA2 Rev 2 March 2005 Instruction Manual PDFSebastian SalazarNo ratings yet
- Physics: Senior Secondary School: SecondDocument53 pagesPhysics: Senior Secondary School: SecondHASSAN OLUMIDENo ratings yet
- NSTSE 2015 Class 9 Answer Key & SolutionDocument7 pagesNSTSE 2015 Class 9 Answer Key & SolutionMota ChashmaNo ratings yet
- WAO in Europe Waste Management 2000Document11 pagesWAO in Europe Waste Management 2000pseunghan9398No ratings yet
- 53-TMSS-03 (Rev 00)Document8 pages53-TMSS-03 (Rev 00)GardellNo ratings yet
- Mhi Integrally Geared CompressorsDocument6 pagesMhi Integrally Geared CompressorscandhareNo ratings yet
- Wet Gas MeterDocument4 pagesWet Gas MeterFareez JamaliNo ratings yet
- SPE 168195 Selectively Shutting Off Gas in Naturally Fractured Carbonate ReservoirsDocument18 pagesSPE 168195 Selectively Shutting Off Gas in Naturally Fractured Carbonate ReservoirsLeopold Roj DomNo ratings yet
- Medical Physics and Biophysics II Course SpecDocument4 pagesMedical Physics and Biophysics II Course SpecGoran MaliNo ratings yet
- EnergeticsqaDocument9 pagesEnergeticsqaArun MuddamsettyNo ratings yet
- Lava Final ReportDocument69 pagesLava Final ReportUma MaheshNo ratings yet
- ZANDER Aufbereitungstechnik GMBHDocument393 pagesZANDER Aufbereitungstechnik GMBHdanenicNo ratings yet
- Solutions to Quiz 3 ProblemsDocument17 pagesSolutions to Quiz 3 ProblemsZahra KhanNo ratings yet
- Aspire Group of Colleges Entry Test PrepDocument3 pagesAspire Group of Colleges Entry Test PrepShahzad AslamNo ratings yet
- DP 1 Datesheet and Test Specifications - TEA 1 2021-2023Document32 pagesDP 1 Datesheet and Test Specifications - TEA 1 2021-2023AaduNo ratings yet
- SNU GraduateCourseCatalogDocument56 pagesSNU GraduateCourseCatalogNick StrikerNo ratings yet
- DACZ 25-160: Operating Instructions Adsorption DryerDocument79 pagesDACZ 25-160: Operating Instructions Adsorption DryerМихайло ЗахаркоNo ratings yet
- PVT ExpressDocument5 pagesPVT ExpressHadi HendizadehNo ratings yet
- Reactivity of Coal Gasification With Steam and CO2Document9 pagesReactivity of Coal Gasification With Steam and CO2udaybhatkandeNo ratings yet
- Lechatlier Real-Life ApplicationsDocument13 pagesLechatlier Real-Life Applicationsapi-28264911680% (5)
- Virial Equation of StateDocument9 pagesVirial Equation of StateSaba ArifNo ratings yet
- Adela Ben-Yakar - Experimental Investigation of Mixing and Ignition of Transverse Jets in Supersonic CrossflowsDocument217 pagesAdela Ben-Yakar - Experimental Investigation of Mixing and Ignition of Transverse Jets in Supersonic CrossflowsWhiteLighteNo ratings yet