Professional Documents
Culture Documents
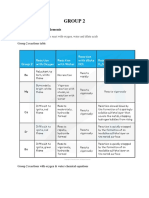
2 Group Two Elements: Beryllium Be Magnesium MG Calcium Ca Strontium SR Barium Ba
Uploaded by
Theodora HamletOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
2 Group Two Elements: Beryllium Be Magnesium MG Calcium Ca Strontium SR Barium Ba
Uploaded by
Theodora HamletCopyright:
Available Formats
Chemistry of the Elements
GROUP TWO ELEMENTS
Be Mg Ca Sr Ba Ra
FIGURE 2.1 Elements In Group Two (2)
Beryllium Be Electronic Structure Electronegativity 1st I.E / KJ mol-1 2nd I.E / KJ mol-1 M.P / oC B.P / C
o
Magnesium Mg [Ne] 2s2 1.2 738 1500 650 1117 0.16 +2 -2.37
Calcium Ca [Ar] 4s2 1.0 590 1100 850 1492 0.20 +2 -2.87
Strontium Sr [Kr] 4s2 1.0 550 1100 770 1367 0.21 +2 -2.89
Barium Ba [Xe] 6s2 0.9 500 1000 710 1673 0.22 +2 -2.90
[He] 2s2 1.5 899 1800 1283 2477 0.11 +2 -1.85
Atomic Radius / nm Oxidation No. Eom+ / mV
TABLE 2.1 Elements In Group Two (2)
2.1
FAJANS RULE
An ionic compound will have a high degree of covalency if: The positive ion is small and highly charged. The negative ion is large (highly polarisable) Generally, covalency is promoted by small cations and large anions.
Copyright Pooran Appadu
Chemistry of the Elements For example, the beryllium ion, Be2+, is so small that it manages to polarize any negative ion to such a degree that its compounds are predominantly covalent. Group II elements are good reducing agents, they give ionic compounds, their oxides and hydroxides are basic and they give hydrogen with oxides. The exception is beryllium. One reason why beryllium is different is that its electrons are not strongly shielded from its nucleus. The radius of the Be2+ ion is extremely small, and it represents a very dense centre of positive charge. Beryllium also has a higher electronegativity than the other elements. Hence, the compounds it makes with non metals should have less ionic character. The solutions of beryllium compounds suffer hydrolysis and are amphoteric rather than completely basic. Beryllium will dissolve in alkali. This is something that magnesium and the other metals in the Group II will not do: Be (s) + 2OH- (aq) BeO22- (aq) + H2 (g)
The product BeO22-, is the berylleto ion, which is better represented in solution as the tetrahydroxoberyllate III ion Be(OH)42-. In its amphoteric behaviour it resembles aluminum in group III.
2.2
REACTIONS OF THE GROUP (II) ELEMENTS
REACTANT Water Be No reaction Mg Slow, rapid with steam. Mg(s) + H2O
(g)
REACTION
MgO(s) + H2 (g)
Ca reacts steadily. Sr and Ba reacts more than Ca Oxygen Dilute Acid All tarnish in air, oxide forms. All burn readily in air. Be, Mg Steadily Ca vigorously St, Ba More vigorous. Halogens Nitrogen All form halides. All form nitrides (except Be). On additon of water nitrides give ammonia. Mg + N2
(s)
(s)
(g)
Mg3N2
(s) (aq)
Mg3N2
TABLE 2.2 Reactions Of The Elements
+ 6H2O Mg(OH)2
+ 2NH3
(g)
2.3
BEHAVIOUR OF OXIDES WITH WATER
The behaviour of the oxides with water:
Copyright Pooran Appadu
Chemistry of the Elements
MgO
CaO
SrO
BaO
Increasing Basic Character
2.4
SOLUBILITIES OF THE SULPHATES
Solubilities of sulphates decrease down the group, also for carbonates, and chromates and hydroxides increases.
SOLUBILITIES AT 25 oC Element Be Mg Ca Sr Ba SO423.79 * 10-1 S1.83 * 10-1 4.66 * 10-3 7.11 * 10-5 9.43 * 10-7 1.3 * 104
CO321
OH-
CrO42-
2.0 * 10-5 1.6 * 10-3 3.3 * 10-2 2.4 * 10-2
8.5 * 101
1.3 * 105
8.7 * 102
7.0 * 106
5.9 * 104
9.0 * 106
1.1 * 106
Decrease Decrease Increase
TABLE 2.3 Solubilities Of Sulphates
Increase
Solubilities are determined by two factors: 1). The ability of crystal lattices to break up. It should be noted that the lattice is very hard to break. HoLatt(D) Highly endothermic.
Copyright Pooran Appadu
Chemistry of the Elements
2).
The release of energy. Much energy is released when the ions are hydrated. HoHyd Highly exothermic. When an ionic compound dissolves in water, the following process occurs: M+A- (s) + (aq) This process is a result of two stages: First: Separation of the ions in the solid: M+A- (s) M+ (g) + A- (g) M+ (aq) + A- (aq)
This is the reverse of lattice formation and requires input of the lattice energy. Second: Involves hydration of the separation ions by water. M+ (aq) + A- (aq)
M+ (g) + A- (g) + (aq)
M+ (g) + A- (g) + (aq)
-HLatt MA(s) = xKJ
-HHyd = -yKJ
M+(aq) + A-(aq)
Hsoln MA(s) = (x-y) KJ = +KJ MA(s) + (aq) Copyright Pooran Appadu
Chemistry of the Elements
FIGURE 2.2 The Relationship Between Lattice Energy, Hydration Energy And Enthalpy Change
THE RELATIONSHIP BETWEEN LATTICE ENERGY, HYDRATION ENERGY AND ENTHALPY CHANGE OF SOLUTION As the ionic radii of M+ and A- increases, the enthalpy change in both of the stages above decreases. The reverse lattice energy process becomes less endothermic and the hydration process exothermic. The cancellation effect of both processes is not always reliable to predict solubilities. The general pattern and trends in the solubility group II salts are: Group II cations containing anions with a charge of -1 (e.g. Cl, NO3-) are generally soluble, except for the hydroxides. Group II cations containing anion with a charge of -2 (e.g. SO42-,CO32-) are generally insoluble, except for some magnesium and calcium salts. The distinct trend of the sulphates: Beryllium and Magnesium (Very Soluble), Calcium (Sparingly Soluble), Strontium and Barium (Insoluble).
2.5
THERMAL DECOMPOSITION OF THE CARBONATES AND NITRATES
The stability if the salt M+A-(s) is dependent on the size of its ions and the charge on these ions. The greater the charge, the stronger is the attraction between the ions and the more stable is M+A- (s). The smaller the ions become, the closer they can approach each other in the solic crystal and the more stable is M+A- (s). Also, when large anions such as CO32- decompose on heating to form smaller anion such as O2-, the crystal containing the latter will generally be more stable. This is because the charge density on the small O2- in will be greater than that on a larger CO32ion, thus O2- will be more strongly attracted to the cations in the crystal and get even closer. NITRATES Group II nitrates and LiNO3 decompose on heating to form their corresponding oxide, e.g. Mg(NO3)2 (s) LiNO3 (s) MgO (s) + 2NO2 (s) + O2 (g) LiO (s) + 2NO2 (s) + O2 (g)
The nitrates achieve greater stability by decomposing to much smaller oxides ion. CARBONATES Group II carbonates and Li2CO3 decomposes to form their corresponding oxide. They achieve thermal stability by forming the O2- ion. MgCO3 (s) Li2CO3 (s) HYRDOXIDE MgO (s) + CO2 (g)
Li2O (s) + CO2 (g)
Copyright Pooran Appadu
Chemistry of the Elements
These decompose to the more stable oxides. Mg(OH)2 (s) MgO (s) + H2O (s)
LiOH decomposes at about 950 oC.
2.6
USES OF MAGNESIUM AND CALCIUM COMPOUNDS
MAGNESIUM AND CALCIUM Calcium Hydroxide (slaked lime). The solution is called lime water. (a) Treatment of fields which are too acidic for healthy plant growth. (b) Mortar a mixture of slaked lime, sand and water. (c) Manufacture of Calcium Hydrogen Sulphate Ca(HSO4)2. The paper industry needs this to remove lignin from woods and leave cellulose, ready to be made into paper. (d) Reaction with chlorine to form bleaching powders, Ca(OCl)2, CaClz. This is a useful source of chlorine, which it liberates readily when an acid is added. CALCIUM OXIDE (a) Neutralizes excessive acidity. (b) Used to dry ammonia. (c) It is refractory, that is, it will not melt even when heated to a very high temperature. MAGNESIUM OXIDE (a) Use as refractory lining in furnaces. CALCIUM CARBONATE (a) Used in the Solvay process for manufacture of sodium carbonate. (b) Used in the iron and steel industry. (c) Used in the glass industry. (d) Used in the cement industry.
Copyright Pooran Appadu
You might also like
- Chapter 11 Group IIDocument7 pagesChapter 11 Group IICharlobabooramNo ratings yet
- The S-Block ElementsDocument55 pagesThe S-Block Elementswealthy58771% (7)
- Group 2 Notes (Sem 2)Document7 pagesGroup 2 Notes (Sem 2)Geethanjali SivakumarNo ratings yet
- 5.3 & 5.4 Group 2: What Is The Outcome From Syllabus?Document34 pages5.3 & 5.4 Group 2: What Is The Outcome From Syllabus?ArichandranMuniandyNo ratings yet
- Chemistry Form 6 Sem 2 04 Notes STPM 2014/2013Document27 pagesChemistry Form 6 Sem 2 04 Notes STPM 2014/2013Raj Nittiya SugumaranNo ratings yet
- Group 2Document32 pagesGroup 2irnihafizan6812No ratings yet
- Chapter 11 - Group IIDocument7 pagesChapter 11 - Group IINabindra RuwaliNo ratings yet
- Chemistry STPM Semester 2 Group 2Document7 pagesChemistry STPM Semester 2 Group 2kumutha83% (6)
- Chapter 10 Group 2Document8 pagesChapter 10 Group 2Vjayan DharmaNo ratings yet
- Group 2 MetalsDocument19 pagesGroup 2 MetalsSelena JayyNo ratings yet
- The S-Block ElementsDocument9 pagesThe S-Block ElementsKamal DeshapriyaNo ratings yet
- Group 2Document15 pagesGroup 2Behzod ShoraimovNo ratings yet
- Group II Elements Physical Properties and ReactionsDocument15 pagesGroup II Elements Physical Properties and ReactionsDoveNo ratings yet
- CIE Chemistry A Level: 10: Group 2Document7 pagesCIE Chemistry A Level: 10: Group 2Shujaat SiddiquiNo ratings yet
- Group 2Document31 pagesGroup 2Shima SenseiiNo ratings yet
- 2.6 NotesDocument6 pages2.6 NotesLisa DentonNo ratings yet
- Group 2A Metals Properties and TrendsDocument10 pagesGroup 2A Metals Properties and TrendsPhượng NguyễnNo ratings yet
- S-Block ElementDocument31 pagesS-Block ElementSiva ChamlingNo ratings yet
- Group Ii Elements: S-Block Elements Because Their Valence (Bonding) Electrons Are in S OrbitalsDocument3 pagesGroup Ii Elements: S-Block Elements Because Their Valence (Bonding) Electrons Are in S OrbitalsAndreea Maria PavelNo ratings yet
- Group 2Document19 pagesGroup 2Muhammad KalimNo ratings yet
- 2.7 The Periodic Table - Groups 2 and 7Document84 pages2.7 The Periodic Table - Groups 2 and 7Listiyaning TiasNo ratings yet
- s-Block Elements ReviewDocument12 pagess-Block Elements ReviewGaurav ChaudharyNo ratings yet
- Module 3Document6 pagesModule 3Daneilla BanksNo ratings yet
- CAIE Chemistry A-Level: 10: Group 2Document6 pagesCAIE Chemistry A-Level: 10: Group 2ahumanbeinginearthNo ratings yet
- 10 - Group 2Document20 pages10 - Group 2Soma Chowdhury RosyNo ratings yet
- S-Block Booklet (W)Document33 pagesS-Block Booklet (W)akjnfdNo ratings yet
- Metals and Non Metals - NotesDocument13 pagesMetals and Non Metals - NotesmittalshivamNo ratings yet
- 22-Properties Period 3 Oxides and Reactions Chlorides With WaterDocument2 pages22-Properties Period 3 Oxides and Reactions Chlorides With WaterNkemzi Elias NzetengenleNo ratings yet
- S-Block ElementsDocument17 pagesS-Block ElementsPiggu SurfersNo ratings yet
- The S-Block ElementsDocument55 pagesThe S-Block ElementsDika Virga SaputraNo ratings yet
- FaziraRazak Group IIADocument58 pagesFaziraRazak Group IIAaieyinHengNo ratings yet
- Group 2 - The Alkaline Earth Metals: AppearanceDocument5 pagesGroup 2 - The Alkaline Earth Metals: AppearanceLourdes Nitro MarasiganNo ratings yet
- Lesson 1Document19 pagesLesson 1saidbiala414No ratings yet
- S Block ElementsDocument4 pagesS Block ElementssubkitsNo ratings yet
- Group II Metals Properties and ReactionsDocument11 pagesGroup II Metals Properties and ReactionsTimothy HandokoNo ratings yet
- Chemistry of Period II 1Document6 pagesChemistry of Period II 1zakNo ratings yet
- Group 2 ElementsDocument9 pagesGroup 2 Elementskevineben006No ratings yet
- S - and P-Block ElementsDocument8 pagesS - and P-Block Elementssameenrashid410No ratings yet
- Corrosion TextDocument40 pagesCorrosion TextAlex PazmiñoNo ratings yet
- Group 1 & 2 MetalsDocument8 pagesGroup 1 & 2 MetalsDaniel BerryNo ratings yet
- Trends in alkaline earth metalsDocument52 pagesTrends in alkaline earth metalsAntonique HeadmanNo ratings yet
- Group 2 Elements Sem 2 ChemistryDocument12 pagesGroup 2 Elements Sem 2 ChemistryChong Yin Ping100% (1)
- Group I Alkali Metals Properties and UsesDocument6 pagesGroup I Alkali Metals Properties and UsesBrian NyagaNo ratings yet
- 3 1 2 Group 2Document2 pages3 1 2 Group 2DecklinNo ratings yet
- SM Chapter 18 PDFDocument25 pagesSM Chapter 18 PDF李承家No ratings yet
- S Block Element (11th)Document11 pagesS Block Element (11th)Gudia kumariNo ratings yet
- Melting points and reactivity of Group 2 metalsDocument6 pagesMelting points and reactivity of Group 2 metalsAnshu MovvaNo ratings yet
- 3 1 2 Group 2Document2 pages3 1 2 Group 2Garret GordonNo ratings yet
- Group 2 ElementsDocument9 pagesGroup 2 ElementsSumaya TraoreNo ratings yet
- CIE Chemistry A Level: 10: Group 2Document7 pagesCIE Chemistry A Level: 10: Group 2Mildred MunatsiNo ratings yet
- S Block Elements ExplainedDocument6 pagesS Block Elements Explainedkeshavjain7No ratings yet
- Spodumene Lial (Sio) Petalite (Lial (Si O), Lepidolite K (Li, Al) (Al, Si, RB) O (F, Oh)Document16 pagesSpodumene Lial (Sio) Petalite (Lial (Si O), Lepidolite K (Li, Al) (Al, Si, RB) O (F, Oh)dannyNo ratings yet
- Alkaline Earth Metals: Properties and UsesDocument20 pagesAlkaline Earth Metals: Properties and UsesMuhammad Saad SaleemNo ratings yet
- CO2 Corrosion NotesDocument9 pagesCO2 Corrosion NotesRony MayrizalNo ratings yet
- Chemistry of Alkaline Earth Metals: Properties, Reactions and UsesDocument29 pagesChemistry of Alkaline Earth Metals: Properties, Reactions and Usessamuel kpamiosaNo ratings yet
- Mcquarrie InterD FinalDocument8 pagesMcquarrie InterD FinalAyush BudhirajaNo ratings yet
- InorganicDocument19 pagesInorganicah_16036566100% (1)
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Extractive Metallurgy 2: Metallurgical Reaction ProcessesFrom EverandExtractive Metallurgy 2: Metallurgical Reaction ProcessesRating: 5 out of 5 stars5/5 (1)
- Human Impact QuestionsDocument2 pagesHuman Impact QuestionsTheodora HamletNo ratings yet
- 01 - Using Circle PropertiesDocument1 page01 - Using Circle PropertiesTheodora HamletNo ratings yet
- CXC English A Exam StacyewfsdDocument3 pagesCXC English A Exam StacyewfsdTheodora HamletNo ratings yet
- 5076 Introduction To EnergeticsDocument1 page5076 Introduction To EnergeticsTheodora HamletNo ratings yet
- 4th Rates RXNDocument1 page4th Rates RXNTheodora HamletNo ratings yet
- 01 - Investigating Properties of CirclesDocument2 pages01 - Investigating Properties of CirclesTheodora HamletNo ratings yet
- Electrochemistry Lesson 15 The Applications of Electrolysis SimhghDocument7 pagesElectrochemistry Lesson 15 The Applications of Electrolysis SimhghTheodora HamletNo ratings yet
- (142496578) 836 Effects of The Haitian RevolutionDocument2 pages(142496578) 836 Effects of The Haitian RevolutionTheodora HamletNo ratings yet
- 6b Lab ManualDocument34 pages6b Lab ManualTheodora Hamlet100% (1)
- What Is Planning-And-Designing (1) GHGHDFDocument7 pagesWhat Is Planning-And-Designing (1) GHGHDFTheodora HamletNo ratings yet
- (85888621) CSEC Food&Nutrition SBAForm PlanSheetHE-5Document5 pages(85888621) CSEC Food&Nutrition SBAForm PlanSheetHE-5Jelani GreerNo ratings yet
- 5076 Introduction To EnergeticsDocument1 page5076 Introduction To EnergeticsTheodora HamletNo ratings yet
- Circle Theorems (Tangents) Great AssistanceDocument14 pagesCircle Theorems (Tangents) Great AssistanceTheodora HamletNo ratings yet
- Circle Theorems (Tangents) Great AssistanceDocument14 pagesCircle Theorems (Tangents) Great AssistanceTheodora HamletNo ratings yet
- Dec, Science Tech HelloDocument10 pagesDec, Science Tech HelloTheodora HamletNo ratings yet
- A Plant CellDocument1 pageA Plant CellTheodora HamletNo ratings yet
- (85888621) CSEC Food&Nutrition SBAForm PlanSheetHE-5Document5 pages(85888621) CSEC Food&Nutrition SBAForm PlanSheetHE-5Jelani GreerNo ratings yet
- Builda Sentence ExprienceDocument2 pagesBuilda Sentence ExprienceTheodora HamletNo ratings yet
- Social Studies Sba 1aDocument8 pagesSocial Studies Sba 1aTheodora Hamlet60% (5)
- 2 Group Two Elements: Beryllium Be Magnesium MG Calcium Ca Strontium SR Barium BaDocument6 pages2 Group Two Elements: Beryllium Be Magnesium MG Calcium Ca Strontium SR Barium BaTheodora HamletNo ratings yet
- 642G Chockfast InstallDocument6 pages642G Chockfast Installgustavofx21No ratings yet
- Mechanics of Composite Materials and Structures by Madhujit Mukhopadhyay 8173714770Document5 pagesMechanics of Composite Materials and Structures by Madhujit Mukhopadhyay 8173714770Jinsan JinsanNo ratings yet
- Seal Non-Structural Cracks Hydrophilic PolyurethaneDocument1 pageSeal Non-Structural Cracks Hydrophilic PolyurethaneFALwilliamsNo ratings yet
- 5 3 Enthalpy Change and Exothermic and Endothermic ReactionsDocument24 pages5 3 Enthalpy Change and Exothermic and Endothermic Reactionsapi-210028385No ratings yet
- DWST-MTHL-QP007 Painting ProcedureDocument31 pagesDWST-MTHL-QP007 Painting ProcedureDeepak UpadhayayNo ratings yet
- Inspection & Testing Requirements: ScopeDocument1 pageInspection & Testing Requirements: ScopeSamiNo ratings yet
- 01 Bldgconst1Document55 pages01 Bldgconst1Lala LeeNo ratings yet
- Joel R. Fried - Solutions Manual For Polymer Science and Technology (2015Document58 pagesJoel R. Fried - Solutions Manual For Polymer Science and Technology (2015chemical_alltime100% (5)
- Astm C494C494M-17Document3 pagesAstm C494C494M-17Indrawan Soleh PutraNo ratings yet
- M30 Concrete Mix DesignDocument8 pagesM30 Concrete Mix DesignImran KhanNo ratings yet
- Copper Alloy Continuous Castings: Standard Specification ForDocument8 pagesCopper Alloy Continuous Castings: Standard Specification ForAccount Jaywant EngineeringNo ratings yet
- Product Guide: Pidilite Industries LTDDocument6 pagesProduct Guide: Pidilite Industries LTDশুভাশীষ গাঙ্গুলীNo ratings yet
- ACSR RABBIT CONDUCTOR TEST REPORTDocument1 pageACSR RABBIT CONDUCTOR TEST REPORTFaizi MNo ratings yet
- Crowd Control Barrier CatalogDocument3 pagesCrowd Control Barrier CatalogtempfencingNo ratings yet
- 06 Science Ch5 Seperation of SubstancesDocument3 pages06 Science Ch5 Seperation of SubstancesReality of PoliticsNo ratings yet
- Butyl Diglycol Acetate ManufacturersDocument4 pagesButyl Diglycol Acetate ManufacturersSomuSolventsNo ratings yet
- W 2 - EarthandLifeScience - q1 - CLAS2 - CommonRockFormingMineralsDocument15 pagesW 2 - EarthandLifeScience - q1 - CLAS2 - CommonRockFormingMineralsPeter Paul PaalanNo ratings yet
- Allectra 12 KF IsoDocument20 pagesAllectra 12 KF Isomsyan1965No ratings yet
- Triacetate FibreDocument3 pagesTriacetate FibreSatadeep DattaNo ratings yet
- Weflo Swing Check Valve F0311-300-Data-SheetDocument1 pageWeflo Swing Check Valve F0311-300-Data-Sheetachmad.zs7827No ratings yet
- No 1Document10 pagesNo 1Yvon BaguioNo ratings yet
- DRUŠTVO TERMIČARA SRBIJE - DESALINATION STORYDocument39 pagesDRUŠTVO TERMIČARA SRBIJE - DESALINATION STORYrakicbgNo ratings yet
- Pick Up The Most Appropriate Statement of The Multiple-Choice Answers by Comment On The Correct AnswersDocument9 pagesPick Up The Most Appropriate Statement of The Multiple-Choice Answers by Comment On The Correct Answersأحمد إبراهيم شواربNo ratings yet
- Microban 9200-200 TDSDocument4 pagesMicroban 9200-200 TDSArsalan AslamNo ratings yet
- Explain What Happens During The Following Stages of Bleaching Chemical PulpsDocument8 pagesExplain What Happens During The Following Stages of Bleaching Chemical PulpszacksNo ratings yet
- Victor Propane LPG Natural Gas Cutting Tip ChartDocument2 pagesVictor Propane LPG Natural Gas Cutting Tip ChartYhamil La MadridNo ratings yet
- Discussion of Extended Drucker-Prager Yield Criterion in Slope Stability AnalysisDocument4 pagesDiscussion of Extended Drucker-Prager Yield Criterion in Slope Stability AnalysisAbdelmoez ElgarfNo ratings yet
- Engage 7467Document2 pagesEngage 7467RomDipaNo ratings yet
- 02L Lubricator - MiniatureDocument1 page02L Lubricator - MiniatureGonzalo AlvarezNo ratings yet
- Pneumatic Structures GuideDocument28 pagesPneumatic Structures GuideAbhi Nand100% (1)