Professional Documents
Culture Documents
4032A2
Uploaded by
raghunagar44Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
4032A2
Uploaded by
raghunagar44Copyright:
Available Formats
AMENDMENT NO.
2 MARCH 2010 TO IS 4032 : 1985 METHOD OF CHEMICAL ANALYSIS OF HYDRAULIC CEMENT
( First Revision ) (Page 4, clause 2.1) Insert the following at the end: 2.2 Preparation of Sample for Analysis Keep about 10 g sample in a large glass Petri dish and spread it evenly into a thin layer. Put it in the oven maintained at 105 5C for one hour to remove any superficially adsorbed moisture. Take it out and keep in a desiccator and cool it to room temperature for chemical analysis. (Page 7, clause 4.1.20) Insert the following at the end: 4.1.21 Nitrobenzene
4.1.23.1 Standardization of 0.025 N silver nitrate solution Silver nitrate may be standardized to check its normality by titrating with a standard solution of 0.025 N sodium chloride.
Take 10 ml of 0.025 N sodium chloride solution in a 250-ml conical flask. Add 2 ml of potassium chromate indicator. Titrate with silver nitrate solution to slight brick red colour end point. The exact normality of silver nitrate may be
Price Group 2 1
No t
Preparation of 0.025 N Sodium Chloride Dry sodium chloride at 600 50C for 50 min and cool in a desiccator. Weigh 1.461 5 g in a 250-ml beaker. Dissolve in about 50 ml water. Transfer in a one-litre volumetric flask and make up to one litre.
for
sa
4.1.23 Silver Nitrate Solution 0.025 N. Dry silver nitrate at 120C for two hours and allow to cool in a desiccator. Weigh out accurately 4.246 7 g in a 250 ml beaker, dissolve in little quantity of distilled water and transfer in a one-litre volumetric flask and make up to the mark.
le
4.1.22 Potassium Chromate Indicator Dissolve 0.2 g in 100 ml of distilled water.
Amend No. 2 to IS 4032 : 1985 calculated using the equation: N1V1 = N2V2 where N1 = normality of sodium chloride that is 0.025, V1 = 10 ml, N2 = normality of silver nitrate solution, and V2 = volume of silver nitrate required. 4.1.24 Ferric Alum Indicator A saturated solution of ammonium iron (III) sulphate dodecahydrate [NH4Fe(SO4)12H2O] in distilled water (about 40 percent) is made, to which few drops of 6 N nitric acid are added. 1 ml of this is used in the titration. 4.1.25 Ammonium Thiocyanate Solution 0.025 N. Weigh accurately 1.903 0 g in a 250-ml beaker, dissolve in about 50 ml water and transfer in a one-litre volumetric flask. Make up to one litre. This solution needs standardization with standard silver nitrate solution. 4.1.25.1 Standardization of ammonium thiocyanate solution Pipette 20 ml of standard 0.025 N silver nitrate solution into a 250-ml conical flask, add 5 ml of 6 N nitric acid and 1 ml of ferric alum indicator. Run in the ammonium thiocyanate solution from a burette. At first a white precipitate is produced, rendering the liquid a milky appearance and as each additional drop of thiocyanate falls in, it produces a reddish-brown cloud, which quickly disappears on shaking. As the end point approaches, the precipitate becomes flocculent and settles easily; finally one drop of the thiocyanate solution produces a faint brown colour, which no longer disappears upon shaking. This is the end point. It is essential to shake vigorously during titration in order to obtain correct results, because the freshly precipitated silver thiocyanate formed during the titration adsorbs silver ions, thereby causing a false end point, which however, disappears with vigorous shaking. Calculate the normality of the thiocyanate solution by using the normality equation:
No t
where
N3V3 = N4V4 N3 = normality of silver nitrate that is 0.025, V3 = 20 ml, N4 = normality of ammonium thiocyanate, and V4 = volume of ammonium thiocyanate required. 2
for
sa
le
Amend No. 2 to IS 4032 : 1985 (Page 7, clause 4.2) Substitute the following for the existing: 4.2 Loss on Ignition Heat about 1 g of oven-dried sample, m1 (accurately weighed up to four decimal places) for 15 min in a weighed and covered platinum crucible (a porcelain crucible may also be used) of 20 to 25 ml capacity by placing it in a muffle furnace at a temperature of 950 25C; cool and weigh. Check the loss in weight by a second heating for 5 min and reweigh (m2). Calculate the loss on ignition as below:
(m1 - m2 ) 100 m1 Round off the calculated value and report up to 1 decimal place.
Percent loss on ignition = (Page 7, clause 4.3.1, line 1) Substitute Transfer about 0.5 g - 1.0 g, W (accurately weighed up to four decimal places) of oven dried sample for Transfer 0.5 g of the sample. (Page 8, clause 4.3.4) Substitute the following for the existing formula:
R2O3, percent =
Weight of residue 100 W
(Page 12, clause 4.7.1.2) Substitute the following for the existing formula:
No t
MgO, percent = where
CaO, percent =
(Page 13, clause 4.8.1.1) Substitute the following for the existing:
W1 36.22 W
W1 = grams of residue (Mg2P2O7), and 36.22 = molecular ratio of 2 MgO to Mg2P2O7 (0.362 2) 100 3
for
Weight of residue 100 W
sa
(W1 - W2 ) 100 W (Page 9, clause 4.4.3.1) Substitute the following for the existing formula:
Silica, percent =
le
Amend No. 2 to IS 4032 : 1985 (Page 21, clause 4.12.3.1) Insert the following at the end: 4.13 Chloride 4.13.1 The sample is boiled with dilute nitric acid, thereby dissolving all the chlorides present. Excess standard silver nitrate solution is added to it. The chlorides react with silver nitrate forming silver chloride. Excess of silver nitrate is then back titrated with standard ammonium thiocyanate solution. Ferric alum is used as an indicator which gives reddish-brown colour at the end point. The reactions involved are: Ag+ + Cl = AgCl Ag+ + SCN = Ag SCN When the reactions are complete, the slightest excess of thiocyanate produces a reddish-brown colouration due to the formation of complex ferric-thiocyanate ion: Fe+++ + SCN = [FeSCN]++
As the reagents and distilled water, etc, used in the exercise may contain some chloride as an impurity, it is essential to subtract this to get the actual chloride content in the sample. For this a blank run is done as given in 4.13.2.2.
No t
4.13.2.1 Dry the sample at 110C for 1 hour and cool in a desiccator. Take 2 g of sample accurately weighed up to fourth decimal place in a 250-ml beaker. Add about 40 ml of distilled water and 10 ml concentrated nitric acid. Heat to boil and shake thoroughly. Keep for few minutes to settle and then filter through Whatman No. 40 filter paper in a conical flask. Wash the filter paper along with its contents 4-5 times with hot distilled water. Reject the insoluble in the funnel. To the filtrate add 2 ml of nitrobenzene and 10 ml 0.025 N silver nitrate solution with the help of a micro burette. Then add 1 ml of ferric alum indicator. Titrate with 0.025 N ammonium thiocyanate solution to get a faint brown colour as described in 4.1.25.1. Note down the volume of ammonium thiocyanate used.
for
sa
4.13.2 Procedure
le
4
Amend No. 2 to IS 4032 : 1985 4.13.2.2 Blank Take 100 ml distilled water in a conical flask. Add 10 ml concentrated nitric acid + 10 ml 0.025 N silver nitrate solution, 2 ml nitrobenzene and 1 ml ferric alum. Titrate with 0.025 N ammonium thiocyanate solution. Let ammonium thiocyanate required be y ml Blank = (10 y) ml 4.13.3 Calculation Let ammonium thiocyanate used in the sample titration be x ml Silver nitrate consumed by the chlorides, Z = [10 (10 y) x ] ml Percent chloride, Cl =
Z 0.035 46 0.025 100 Mass of sample
4.13.4 Repeatability and Reproducibility The standard deviation of repeatability of the method is 0.005 percent. The standard deviation of reproducibility is 0.010 percent. (Page 34, clause 6.12.3) Insert the following at the end: 6.13 The method for determining chloride content in Portland slag cement shall be the same as described in 4.13.
7.5 The method for determining chloride content in Portland slag cement shall be the same as described in 4.13.
(CED 2)
Reprography Unit, BIS, New Delhi, India
No t
(Page 36, clause 7.4) Insert the following at the end:
for
sa
le
5
NOTE The calculation is based on the normalities of silver nitrate and ammonium thiocyanate to be exactly same, that is, 0.025. In case the normalities differ, then correction may be applied using appropriate factor.
You might also like
- Is SP 23 1982 PDFDocument151 pagesIs SP 23 1982 PDFMano MaddulaNo ratings yet
- 455 2015 Portland Slag CementAMD1 Reff2020Document14 pages455 2015 Portland Slag CementAMD1 Reff2020Debabrata PalNo ratings yet
- Draft Indian Standard: Bureau of Indian StandardsDocument9 pagesDraft Indian Standard: Bureau of Indian StandardsPavan KumarNo ratings yet
- DS - 645 - Pro Anticarb HB Elastomeric CoatingDocument2 pagesDS - 645 - Pro Anticarb HB Elastomeric CoatingRay EngineeringNo ratings yet
- Prevention of Frost DamageDocument18 pagesPrevention of Frost DamageBoris DikovNo ratings yet
- Is 16651-2017Document18 pagesIs 16651-2017Assistant Coordinator Business DevelopmentNo ratings yet
- 2021 Annex C &DDocument24 pages2021 Annex C &DSaurav Kumar100% (1)
- Amendment No. 4 July 2019 TO Is 1786: 2008 High Strength Deformed Steel Bars and Wires For Concrete Reinforcement - SpecificationDocument2 pagesAmendment No. 4 July 2019 TO Is 1786: 2008 High Strength Deformed Steel Bars and Wires For Concrete Reinforcement - SpecificationOMEGA CONSULTANT SERVICESNo ratings yet
- 90th Percentile CBR 4.5 Borrow Area CBR 14.2Document4 pages90th Percentile CBR 4.5 Borrow Area CBR 14.2Akshay Aithal KandoorNo ratings yet
- Is 15388 (2003) - Specification For Silica FumeDocument13 pagesIs 15388 (2003) - Specification For Silica FumeN GANESAMOORTHYNo ratings yet
- JSW Ggbs 1 2015 PDFDocument1 pageJSW Ggbs 1 2015 PDFMohammed Javeed ahmedNo ratings yet
- K Value DetailsDocument10 pagesK Value Detailskishor150688100% (1)
- Renderoc RGDocument3 pagesRenderoc RGR.ThangarajNo ratings yet
- Is 3812 2 2003Document14 pagesIs 3812 2 2003VijayKatariaNo ratings yet
- Is 383 (1970) Grading ZonesDocument2 pagesIs 383 (1970) Grading ZonesAnonymous 2RduvkjgZNo ratings yet
- Use of Waste Plastic in Bituminous Concrete MixDocument51 pagesUse of Waste Plastic in Bituminous Concrete MixRahul KumarNo ratings yet
- Presented by - Aniket Ghosh Dastidar Construction Engg. 4 Year Jadavpur UniversityDocument20 pagesPresented by - Aniket Ghosh Dastidar Construction Engg. 4 Year Jadavpur UniversitySaurabh AgrawalNo ratings yet
- Scheme of Testing and Inspection For Certification of Carbon Steel Cast Billet Ingots Billets Blooms and Slabs For Re Rolling Into Seel For General Structural Purpose PDFDocument6 pagesScheme of Testing and Inspection For Certification of Carbon Steel Cast Billet Ingots Billets Blooms and Slabs For Re Rolling Into Seel For General Structural Purpose PDFbipinagarwalNo ratings yet
- Amendment No. 5 July 2019 TO Is 456: 2000 Plain and Reinforced Concrete - Code of PracticeDocument7 pagesAmendment No. 5 July 2019 TO Is 456: 2000 Plain and Reinforced Concrete - Code of PracticeRajan GuptaNo ratings yet
- 460 (Part-1)Document15 pages460 (Part-1)rambinod67% (3)
- IS 4031 - Part15Document8 pagesIS 4031 - Part15Nagaraju ChintaNo ratings yet
- 04a Physical PropertiesDocument13 pages04a Physical PropertiesGanesh.RajanNo ratings yet
- Masterset Ac 100: Non-Chloride Hardening Accelerator For ConcreteDocument2 pagesMasterset Ac 100: Non-Chloride Hardening Accelerator For Concretemehrdad_so1981100% (1)
- Indian Standard (First Revision) : Method of Chemical Analysis of Hydraulic CementDocument44 pagesIndian Standard (First Revision) : Method of Chemical Analysis of Hydraulic CementArijit dasguptaNo ratings yet
- 3812 1 PDFDocument14 pages3812 1 PDFइंजि कौस्तुभ पवारNo ratings yet
- Is 4082Document14 pagesIs 4082Priyanka MishraNo ratings yet
- Fly Ash ClasificationDocument15 pagesFly Ash ClasificationprabhuwbNo ratings yet
- Is 2547Document11 pagesIs 2547Vivek GosaviNo ratings yet
- 2386 (Part-V) PDFDocument11 pages2386 (Part-V) PDFSoundar PachiappanNo ratings yet
- Is 228 (Part1) - Methods For Chemical Analysis of Steels - Determination of Carbon by Volumetric MethodDocument4 pagesIs 228 (Part1) - Methods For Chemical Analysis of Steels - Determination of Carbon by Volumetric Methodjjosej100% (1)
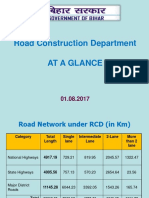
- RCD Bihar at A Glance 2017Document147 pagesRCD Bihar at A Glance 2017HR NagalandNo ratings yet
- IS 2542 - 1978 (Part-2-Sec1 of 12) Method of Test For Gypsum Plaster, Concrete & Product-Gypsum Product PDFDocument27 pagesIS 2542 - 1978 (Part-2-Sec1 of 12) Method of Test For Gypsum Plaster, Concrete & Product-Gypsum Product PDFAnilNo ratings yet
- IS 456 2016 Google SearchDocument2 pagesIS 456 2016 Google Searchvasudeo_eeNo ratings yet
- Is 17372 2020Document12 pagesIs 17372 2020lovehackinggals100% (1)
- Is 1448 16 1990 PDFDocument10 pagesIs 1448 16 1990 PDFDario de SantiagoNo ratings yet
- Alccofine 1106 TDS V2Document3 pagesAlccofine 1106 TDS V2Siddhesh Kamat MhamaiNo ratings yet
- 1489 2 PDFDocument15 pages1489 2 PDFshashikant gaurNo ratings yet
- Is 4020 PDFDocument34 pagesIs 4020 PDFAnand Kumar100% (1)
- Microfine Ordinary Portland Cement - Specification: Indian StandardDocument18 pagesMicrofine Ordinary Portland Cement - Specification: Indian StandardDevesh Kumar PandeyNo ratings yet
- Mix Design FormatDocument3 pagesMix Design FormatAkshay MitraNo ratings yet
- OPC-53 TC KDP For 6th Week 2020 (7 Days) PDFDocument1 pageOPC-53 TC KDP For 6th Week 2020 (7 Days) PDFSahul Ahameed100% (1)
- Mix Design For PQCDocument34 pagesMix Design For PQCSAMRADDHI PRAJAPATINo ratings yet
- C1582C1582M-Aditivos Inhibidores de CorrosionDocument10 pagesC1582C1582M-Aditivos Inhibidores de CorrosionAnthony López HuamanNo ratings yet
- Tractor Emulsion AdvancedDocument2 pagesTractor Emulsion Advancedsatsanh_22100% (1)
- Shear Strength of Expansive SoilsDocument8 pagesShear Strength of Expansive SoilsSanko KosanNo ratings yet
- IRC SP 98-2020 Final For EmailDocument18 pagesIRC SP 98-2020 Final For EmailcricketloversiitNo ratings yet
- Conplast WL Xtra: Integral Waterproofi NG Liquid Admixture For Concrete, Plaster and MortarsDocument2 pagesConplast WL Xtra: Integral Waterproofi NG Liquid Admixture For Concrete, Plaster and MortarsPrasantaNo ratings yet
- BS 1881-116-1983 - Testing Concrete Part 116 - Method For Determination of Compressive Strength of Concrete CubesDocument10 pagesBS 1881-116-1983 - Testing Concrete Part 116 - Method For Determination of Compressive Strength of Concrete CubesSarifNo ratings yet
- Is 13311.2.1992 PDFDocument12 pagesIs 13311.2.1992 PDFTayyab AnwarNo ratings yet
- Is 16720 Product Manual Fuel AshDocument7 pagesIs 16720 Product Manual Fuel AshKolkata PIUNo ratings yet
- Disclosure To Promote The Right To InformationDocument17 pagesDisclosure To Promote The Right To Informationchannakeshava pandurangaNo ratings yet
- Is 1727 1967Document55 pagesIs 1727 1967VijayKataria0% (1)
- CAC-Hyperfluid R150: Superplasticiser For High Performance Concrete Based On Poly-Carboxylic EtherDocument2 pagesCAC-Hyperfluid R150: Superplasticiser For High Performance Concrete Based On Poly-Carboxylic Ethermohsin shaikh100% (1)
- EN 12350-11 (2010) Testing Fresh Concrete - Self-Compacting Concrete - Sieve Segregation TestDocument14 pagesEN 12350-11 (2010) Testing Fresh Concrete - Self-Compacting Concrete - Sieve Segregation TestmariannydanimartinezNo ratings yet
- Is: 383-2016Document21 pagesIs: 383-2016yurendraNo ratings yet
- IS 1786 Amed 3Document3 pagesIS 1786 Amed 3Vikash MudgilNo ratings yet
- Is 5522 1992Document6 pagesIs 5522 1992psewagNo ratings yet
- Amendment No. 2 March 2010 TO Is 4032: 1985 Method of Chemical Analysis of Hydraulic CementDocument5 pagesAmendment No. 2 March 2010 TO Is 4032: 1985 Method of Chemical Analysis of Hydraulic CementThetarunNo ratings yet
- 717 Na OHEDocument7 pages717 Na OHEGlüklich SonneNo ratings yet
- Sop QC VSDocument13 pagesSop QC VSmuzzumilNo ratings yet
- Not For Sale: Amendment No.2 November 1991 Is 8042: 1989 White Portland Cement-SpecificationDocument1 pageNot For Sale: Amendment No.2 November 1991 Is 8042: 1989 White Portland Cement-SpecificationRaghu NagarNo ratings yet
- Assesor Training-2Document4 pagesAssesor Training-2Raghu Nagar100% (1)
- Andi 4.7G Cobalt: Gyro Sensor, G - Sensor, Proximity Sensor, Light Sensor, Magnetic SensorDocument2 pagesAndi 4.7G Cobalt: Gyro Sensor, G - Sensor, Proximity Sensor, Light Sensor, Magnetic SensorRaghu NagarNo ratings yet
- ASTM Reference OilsDocument25 pagesASTM Reference OilsRaghu NagarNo ratings yet
- Aimil 390Document1 pageAimil 390Raghu NagarNo ratings yet