Professional Documents
Culture Documents
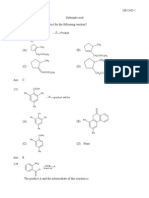
(Mazza 1933) - PH: J. J. and H. Macy and and
Uploaded by
Swati2013Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
(Mazza 1933) - PH: J. J. and H. Macy and and
Uploaded by
Swati2013Copyright:
Available Formats
THE ACTION OF MICROORGANISMS ON FATS
I. OXYGEN UPTAKE BY BACTERIA IN THE PRESENCE OF LIPID SUBSTRATES1
J. J. JEZESKI,' H. 0. HALVORSON,S AND H. MACY
Divisions of Bacteriology and Dairy Husbandry, University of Minnesota, Minneapolis and
St. Paul, Minnesota
Received for publication January 30, 1950
1 Taken from data presented in a thesis submitted to the Graduate Faculty of the University of Minnesota by J. J. Jezeski in partial fulfillment of the requirements for the degree
of Doctor of Philosophy.
This work was supported in part by a grant from the National Institute of Health,
Division of Research Grants and Fellowships.
Scientific Journal Series, Paper No. 2506, Minn. Agr. Expt. Sta.
2 National Institute of Health Predoctorate Research Fellow.
'Present address: Department of Bacteriology, University of Illinois, Urbana, Illinois.
645
Downloaded from http://jb.asm.org/ on December 26, 2014 by guest
The ability of many bacteria to hydrolyze fats has been well established.
On the other hand, the oxidative action of bacteria on this type of substrate
has not been so well investigated. Both chemical and cell respiration studies
have demonstrated that bacteria can oxidize fats, but relatively little information has been obtained on the characteristics of this action on lipid substrates.
Chemical evidence of bacterial oxidation has been obtained on olive oil (Pigulewski and Chaxik, 1929), soybean oil (Horowitz-Wlassova and Livschitz, 1935),
hardened cottonseed oil and leaf lard (Jensen and Grettie, 1933, 1937), and
triolein (Castell and Garrard, 1941). Corn oil was used as the substrate in the
Warburg and Thunberg techniques by Mundt and Fabian (1944). A comparison
of the results revealed no agreement between the two methods. The Thunberg
technique was used by Quastel and Whetham (1925) to show that B. coli-communis could dehydrogenate the lower fatty acids. The same species was able to
oxidize the sodium salts of stearic, oleic, and palmitic acids in the Warburg
apparatus (Mazza and Cimmino, 1933). At pH 7.5 stearate was oxidized at the
greatest rate, followed by oleate and palmitate. These results were confirmed
by Singer and Barron (1945), who also showed that the oxidative enzyme contained active SH groups. Barron and Friedemann (1941) demonstrated that
several cultures of bacteria not capable of fermenting glucose were able to
oxidize acetate, jpropionate, and butyrate, and that more than one enzyme might
be responsible for the oxidation of saturated fatty acids. Streptococcus mitis
oxidized butyrate aerobically with the accumulation of H202 (Niven et al.,
1945).
The purpose of this study was to gather additional information concerning
the metabolic response of various bacterial cultures on lipid substrates by use of
the Warburg technique.
646
J. J.
JEZESKI, H. 0. HALVORSON, AND H. MACY
[vol. 59
METHODS
RESULTS
A number of cultures isolated from various sources were tested for their
ability to take up increased amounts of oxygen in the presence of fatty substrates.
Downloaded from http://jb.asm.org/ on December 26, 2014 by guest
Oxygen utilization was measured by means of Warburg constant volume
respirometers of the single side arm type. Conventional techniques (Umbreit et
al., 1945) were followed in all experiments. The fluid volume was 3.2 ml consisting
of 1.0 ml buffer, 1.0 ml cell suspension, 0.5 ml distilled water, and 0.5 ml substrate
in solution or suspension in distilled water in the main body of the flask; while
the center well contained a strip of filter paper moistened with 0.2 ml of 20 per
cent KOH to absorb the C02 developed during the oxidation. The flasks were
shaken at the rate of 120 oscillations per minute and the temperature of the
water bath was 30 1 0.05 C.
The bacteria were grown on agar in Roux type bottles. The medium consisted
of 0.3 per cent beef extract, 0.3 per cent yeast extract, 0.5 per cent peptone, and
1.5 per cent agar. The reaction was adjusted between pH 6.8 and 7.0. After 24
hours of incubation at temperatures appropriate for each organism, the cultures
were harvested by washing the agar surface of each bottle twice with 5 ml of
chilled salt solution at pH 7.8, prepared according to Landy and Dicken (1942).
These cells were then centrifuged and washed four times, after which the final
suspensions were made up to 30 times the volume of the packed cells with the
chilled salt solution. After such treatment it was found that pre-experimental
aeration did not significantly reduce cellular respiration. The cell preparations
were stored in the refrigerator at 3 to 5 C for periods not longer than 3 days,
except for certain experiments.
Natural substrates used in these experiments were butter oil, cottonseed oil,
and corn oil that had been caustic-refined to remove any traces of free fatty
acids. The saturated fatty acid substrates, which included the free fatty acids,
methyl esters, and triglycerides, were obtained from the Eastman Kodak Company, Chemical Sales Division. These compounds were used without being subjected to any further purification. The methyl esters of oleic and linoleic acids
were obtained from the Hormel Institute, University of Minnesota, at Austin,
Minnesota. The original peroxide values were less than 1.0 for methyl oleate and
between 4.0 and 5.0 for methyl linoleate.
Soaps of the saturated fatty acids were prepared by neutralizing small amounts
of the fatty acids with M/2 NaOH. The reaction was then adjusted to pH 7.5 to
7.8 before the mixture was diluted to get the concentration of soap desired.
In the case of substrates that were insoluble in water, emulsification was
necessary in order to expose an ample substrate surface for enzyme action.
These insoluble substrates were suspended in distilled water by passing the mixture through a hand homogenizer four times. The resulting emulsion was relatively stable for the period of the experiments and usually for a much longer
period. Each substrate was used at a concentration in excess of that required to
produce maximum oxygen uptake with a given suspension of cells.
1950]
ACTION OF MICROORGANISMS ON FATS
647
Downloaded from http://jb.asm.org/ on December 26, 2014 by guest
Of the 44 cultures tested, 30 were able to increase their respiration in the presence
of fat and phosphate buffer (pH 7.0, M/90 final concentration).
From these cultures, four, of differing morphological or physiological characteristics, were selected for detailed study. These included Mycobacterium phlei
(MlOa), an orange-pigmented micrococcus (B4) isolated from spoiled canned
bacon, and two cultures belonging to the genus Pseudomonas (P70 and P78),
which had been isolated from defective butter. The latter two cultures possessed
the reactions of atypical Pseudomonas fluorescens strains and differed between
themselves only in the ability to hydrolyze butterfat. The presence of a lipase
was detected by the use of Nile blue sulfate and butterfat prepared according to
Knaysi's method as cited by Stark and Scheib (1936). Cultures P78 and B4
exhibited very strong lipolytic action, P70 possessed weak lipolytic ability, and
Ml0a was not able to hydrolyze butterfat according to the method given above.
The effect of the chemical composition of the buffer. Experiments with enzymes
of animal origin capable of oxidizing fatty substrates have indicated that the
type of buffer and the presence of inorganic phosphate may influence the rate
of oxidation (Lehninger, 1945a,b). In respiration experiments in which intact
cells are used, the choice of buffers is limited to those of inorganic nature that
could not serve as oxidizable substrates. Thus, acetate, citrate, and glycine
buffers were eliminated immediately. Borate, phosphate, and bicarbonate buffers
at M/60 final concentration were used in these experiments with coconut oil and
corn oil serving as substrates. Potassium bicarbonate was substituted for the
potassium phosphate in the chilled salt solution used in the preparation of the
cell suspensions.
Table 1 presents the results of these comparisons. It may be observed that
phosphate and bicarbonate buffers produced quite similar rates of oxygen uptake
with each substrate; however, in several instances the results with bicarbonate
were slightly lower. The significance of the differences is somewhat doubtful.
The use of borate buffer resulted in considerable inhibition of the oxidation
of these natural triglycerides by all cultures; however, B4 was inhibited to a
lesser extent. The reason for the borate inhibition is unknown, although it has
been suggested that it may be due to interference with the phosphorylation
process.
Effect of phosphate buffer concentration on the rate of oxidation of coconut oil.
In early experiments it was observed that there was considerable change in pH
during the course of the experiments when diluted buffers (M/60 final concentration) were used. In order to reduce the pH shift to a minimum, increased
concentrations of buffer were used. Table 2 summarizes the effect of varying the
phosphate buffer concentration on the rate of oxygen uptake in the presence of
coconut oil.
Cell suspensions of P78 and B4 showed similar responses in that they appeared to be inhibited by increased buffer concentrations. Culture P78 appeared
to be much more sensitive since strong inhibition was demonstrated against
freshly prepared suspension. On aging in the cold this inhibitory effect was increased up to the point where no oxygen was taken up by the substrate at the
648
J. J.
JEZESKI,
H.
[vol. 59
0. HALVORSON, AND H. MACY
M/6 buffer concentration. It should be noted that cultures B4 and P78 possess
strong lipolytic activities compared with the other two cultures tested.
Cultures P70 and MlOa were grouped together by reason of their similar
behavior. With freshly prepared suspensions, the rate of oxidation increased as
the buffer concentration became greater. It would appear that the rate of oxidation was somewhat dependent upon the phosphate concentration, for the fresh
TABLE 1
MICROLITERS 01 OXYGEN CONSUMED IN PRESENCE OF
CULTURE
MlOa
P70
P78
B4
Borate buffer
Bicarbonate buffer
Corn oil
Coconut oil
138
_
13
64
166
111
39
60
Phosphate buffer
Corn oil
Coconut oil
Corn oil
Coconut oil
195
257
236
217
77
178
178
90
297
221
272
101
72
Data are corrected for endogenous respiration.
Time, 90 min; buffer, pH 8.0, M/60 flask conc.; substrate, 10 mg per flask.
TABLE 2
The effect of concentration of buffer on the oxygen consumption in the presence
of coconut oil
MICROLITERS OF OXYGEN CONSUMD IN THE PRESENCE Ol
CULTURE
MlOa
P70
P78
B4
SUBSTRATE
. /6 buffer
(final conc.)
Coconut oil
None*
Coconut oil
None*
Coconut oil
None*
Coconut oil
None*
155
27
122
48
78
27
108
13
(final conc.)
x/12 buffer
x/24 buffer
(final conc.)
K/48 buffer
(final conc.)
135
80
92
117
108
98
127
106
103
16
103
62
177
41
142
9
Time, 90 min; buffer (Clark's phosphate), pH 7.8; substrate, 10 mg per flask (cells
diluted to 1/60 in this experiment).
* Endogenous respiration.
culture at least. As the suspensions were aged in the cold, this relationship
gradually disappeared.
The effect of the composition of natural and pure triglycerids on oxygen consumption. There are indications in the data previously presented that the type of
fat influences the oxygen uptake in a given bacterial culture. Thus, butter, corn,
and coconut oils were compared as substrates in the presence of phosphate buffer.
Butter oil contains large amounts of oleic and the saturated, long-chain (C14 to
Downloaded from http://jb.asm.org/ on December 26, 2014 by guest
The effect of the chemical composition of the buffer on the oxygen uptake of
several cultures in the presence of natural fat substrates
1950]
649
ACTION OF MICROORGANISMS ON FATS
C18) fatty acids and is also relatively rich in the short-chain saturated acids,
whereas coconut oil contains mostly saturated, long-chain (Cn to C16) fatty acids.
Corn oil is composed of a very large percentage of the C18 unsaturated fatty
acids, oleic and linoleic.
The data are summarized in table 3. It is evident in all cases that the coconut
oil was oxidized at a greater rate than either corn or butter oil. The oxygen
uptakes with corn and butter oil were almost identical in the case of three culTABLE 3
cultures of bacteria
MICROITERS O OXYGEN CONSUMED IN PRESENCE O
______________________
CULTURE
Corn oil
Butter fat
MlOa
P70
P78
B4
Coconut oil
352
75
389
157
274
68
270
86
44
293
91
Data are corrected for endogenous respiration.
Time, 90 min; buffer (Clark's phosphate), pH 7.8, M/60, flask conc.; substrates, 10 mg
per flask.
TABLE 4
A comparison of oxygen uptake rates on various triglycerides
MICRtOLTRS 01 OXYGEN CONSUMED
MlOa
Culture .................
P70
Final buffer conc.........
x/48
m/6
x/48
Substrate
Triacetin ........
Tributyrin .......
Trilaurin ........
Tristearin .......
Coconut oil......
196
108
129
29
138
216
267
163
37
277
194
124
111
11
112
B4
P78
X/6
51
-45
118
118
128
M/48
95
-24
241
1
233
X/6
-33
-44
-11
-12
-12
x/48
m/6
22
-3
174
7
148
-35
-34
-9
4
-4
Data are corrected for endogenous respiration.
Time, 90 min; buffer (Clark's phosphate), pH 7.8; substrates, 25 mg per flask.
tures; the exception was culture P70, with which corn oil caused a significantly
greater uptake than butter oil. Even though corn oil and butter oil both contain
large amounts of unsaturated acids, other explanations for their similar behavior
as substrates are not excluded by the data obtained.
Four pure triglycerides of fatty-acid carbon-chain lengths varying from 2
to 18 carbon atoms were also used as substrates. Two buffer concentrations were
employed to determine whether the changes observed on coconut oil also occurred
with the various pure triglycerides. The data are presented in table 4. In the
presence of M/48 buffer the strongly lipolytic cultures B4 and P78 again behaved
Downloaded from http://jb.asm.org/ on December 26, 2014 by guest
The influence of the type of natural fat on the oxygen consumption of four
650
J. J.
JEZESKI, H. 0. HALVORSON, AND H. MACY
[vol. 59
Oxidation of saturated fatty acid esters. Methyl and ethyl esters of the saturated
fatty acids were used in further substrate specificity studies. Results obtained
with methyl alcohol and ethyl alcohol controls demonstrated that the action of
an esterase was unimportant in influencing the results obtained, since none of
these cultures was able to cause a significant uptake of oxygen with either of
these alcohol substrates. The data are presented in table 5. The four cultures
responded in a similar manner toward most of the substrates used. In general,
it appears that there may be two enzyme systems responsible for the oxidation
of these fatty acid esters. As the carbon chain of the fatty acid increased in
length, some oxygen consumption was observed with methyl acetate but little
or no oxidation was observed with the butyric and caprylic esters. The oxygen
consumption rates then increased to a maximum with esters from lauric to
palmitic and fell again with the use of the stearic ester. Thus, in the presence of
M/48 phosphate buffer, two maxima were observed-one with the short-chain
esters, usually acetic, and one with the longer-chain esters, lauric, myristic, or
palmitic.
Downloaded from http://jb.asm.org/ on December 26, 2014 by guest
similarly. Triacetin was weakly or moderately attacked, but there was no oxidation and even some inhibition of endogenous respiration in the presence of tributyrin. Trilaurin was oxidized at a vigorous rate, but no significant amount of
oxygen was taken up in the presence of tristearin. P70 and Ml0a followed an
identical pattern in oxidizing these substrates. Triacetin was oxidized at the
greatest rate. The rate decreased as the length of the carbon chain in the component fatty acid increased until tristearin was only weakly attacked. The use
of M/6 buffer elicited the same response from these organisms on pure triglycerides as on coconut oil. The concentrated buffer stopped oxygen consumption in
the presence of the triglyceride with cultures B4 and P78; and in several instances
inhibition of endogenous respiration was evident. The results with P70 are not
so clean-cut since there was some inhibition with triacetin and tributyrin as substrates, whereas none was shown with trilaurin, tristearin, and coconut oil. An
increase in oxygen uptake due to increased buffer concentration was observed for
culture MlOa in the presence of all substrates.
There is the possibility that glycerol arising from lipase action may be the
oxidizable substrate in these experiments. Glycerol and coconut oil. were compared as substrates for the four cultures. Glycerol was oxidized very vigorously
by culture B4, to a lesser extent by P70 and Ml0a, and relatively weakly by
P78. A comparison showed that glycerol was oxidized by P78 at about onefourth and by P70 and Ml0a at approximately one-half the rates observed with
coconut oil. On the other hand, B4 oxidized glycerol at twice the rate of coconut
oil. Significant oxygen consumption due to glycerol produced when triglycerides
were used as substrates could be eliminated in the case of MlOa due to the
absence of active lipase and with P78 because of a low rate of oxidation of glycerol.
The results obtained with natural fats and pure triglycerides indicate that
the composition of these substrates significantly influences oxygen consumption
by the cultures studied. However, no regular order of substrate specificity could
be determined.
1950]
651
ACTION OF MICROORGANISMS ON FATS
The use of M/6 phosphate buffer produced marked inhibition of oxygen consumption with most of the substrates in the case of culture P78, but culture P70
was affected only when propionate and caprylate esters were used as substrates.
TABLE 5
A comparison of oxygen uptake rates on various saturated fatty acid esters
MICROIITERS OF OXYGEN CONSUMED
B4
MlOa
x/48
m/48
m/6
ii/48
x/6
m/48
196
154
34
6
115
202
115
79
144
120
108
20
19
182
98
156
38
176
108
30
21
-62
149
144
76
81
144
229
203
120
-35
249
273
183
151
283
26
10
15
-37
-19
-12
-24
-21
-14
66
4
31
-5
23
32
13
-6
202
P78
P70
Substrate
Methyl acetate ...................
Methyl propionate ................
Methyl butyrate ..................
Methyl caprylate .................
Ethyl laurate .....................
Ethyl myristate ..................
Methyl palmitate .................
Methyl stearate ......... .........
Coconut oil .......................
Data are corrected for endogenous respiration.
Time, 90 min; buffer (phosphate), pH 7.8; substrates, 20 mg per flask.
TABLE 6
A comparison of rates of oxygen consumption in the presence of sodium soaps
of saturated fatty acids
MICIRLITElLS OF OXYGEN CONSUXED
B4
P78
Culture....................................
MlOa
P70
Final buffer conc..............................
x/48
m/48
m/48
Substrate
Sodium acetate .......... .......
Sodium propionate ..............
Sodium butyrate .................
Sodium caproate ................
Sodium caprylate ...............
Sodium caprate .................
Sodium laurate ..................
Sodium myristate ...............
Sodium palmitate ...............
Sodium stearate .................
108
69
124
133
276
219
-41
255
260
153
546
327
104
184
302
292
286
403
320
369
-23
64
32
79
120
117
102
93
61
52
x/6
x /48
-17
-16
-21
-19
-21
-18
-12
-2
-27
-19
18
10
4
14
19
-16
-18
12
9
-10
Data are corrected for endogenous respiration.
Time, 90 min; buffer (Clark's phosphate), pH 7.8; substrates, 0.033 M, flask conc.
The results obtained with culture B4 indicated a very low activity toward
these esters as compared with glycerol, natural fat, or pure triglyceride (trilaurin).
In addition, it was the only culture tested that did not oxidize ethyl laurate at
the same rate as trilaurin or coconut oil. These observations plus the fact that
Downloaded from http://jb.asm.org/ on December 26, 2014 by guest
Culture .....................................
Final buffer conc................................
652
J. J.
[vol1. 59
JEZESKI, H. 0. HALVORSON, AND H. MACY
glycerol was utilized much faster than coconut oil by this culture is evidence that
the oxygen consumption of this organism in the presence of fat was due principally to the oxidation of glycerol produced by the action of lipase.
Oxidation of the sodium salts (soaps) of the saturated fatty acids. The form of the
substrate may be important in affecting the rate of oxygen consumption in these
experiments. This is apt to be true particularly if the form of fatty acid substrate
is changed from an ester to a sodium salt, since some of the physical and chemical
properties of these compounds are quite different. Homogenizing an ester in
MICROLITERS OF OXYGEN CONSUMED AT
CULTURE
pH 5.8
pH 6.1
p16.5
pH 7.0
pH 7.5
pH 7.8
pH 8.0
102
99
91
91
102
95
101
82
102
107
94
112
47
89
64
102
64
135
130
249
245
273
276
74
89
Methyl acetate
MlOa
P70
P78
B4
126
118*
84
38*
98
61*
73
46*
111
88
81
77
104
86
80
26
78
34
70
39
106
96
91
Ethyl laurate
MlOa
P70
P78
B4
112
125
86*
218
173*
232
233
178*
76
55*
81
252
117
72
238
175
262
207
71
38
168
143
251
169
279
246
93
84
122
226
274
85
Time, 90 min; buffer (Clark's phosphate), M/48, flask conc.; substrates, 0.033 M, flask
conc.
* All
similar values corrected for endogenous,respiration.
distilled water produces an emulsion, whereas the sodium salts produce either
true solutions or colloidal solutions depending on the fatty acid involved.
Table 6 presents the data obtained on the activity of these cultures toward
the sodium salts of the saturated fatty acid series. There is little question that
the sodium salts were utilized to much the same extent as the methyl esters,
with, of course, a few individual exceptions. There is not, however, the consistency of results that is obtained with the methyl esters nor are the two maxima
in rate of oxygen uptake so apparent. Whereas with the esters the two maxima
were fairly well defined with all cultures, the results on the sodium salts showed
Downloaded from http://jb.asm.org/ on December 26, 2014 by guest
TABLE 7
The influence of pH of buffer on oxygen consumption of several cultures in the
presence of saturated fatty acid esters
1950]
653
ACTION OF MICROORGANISMS ON FATS
TABLE 8
The influence of pH of buffer on oxygen constumption in the presence of pure
triglycerides and glycerol
MICROLITERS OF OXYGEN CONSUMED AT
CULTURE
SUBSTRATE
MlOa
Triacetin
P70
Triacetin
P70
Trilaurin
P70
Glycerol
pH 5.8
pH 6.2
pH 6.6
pH 7.0
pH 7.4
pH 7.6
pH 7.9
38
24*
155
116*
192
159*
98
55
80
70
170
122
261
216
110
57
114
120
201
220
271
286
131
174
130
113
269
212
317
251
219
132
129
111
296
216
362
275
253
145
67*
167
244
96
Time, 90 min; buffer (Clark's phosphate), m/48, flask conc.; substrates, 0.033 M, flask
conc.
* All similar values corrected for endogenous respiration.
the maximum rate of oxygen consumption took place in the vicinity of pH 7.4
to 7.8 with culture B4, and pH 7.0 to 8.0 with P78. Relatively uniform activity
was recorded for P70 over the pH range studied. A rather sharp maximum at pH
7.8 was shown by MlOa. Thus for this latter organism, at least, this is evidence
that the same enzyme does not attack acetate and laurate and that several enzymes may act on substrates of the saturated fatty acid series.
Table 8 presents a comparison of several pure triglycerides and glycerol as
substrates at pH levels similar to those previously used. These results include
data obtained on only two cultures, MlOa and P70. The data indicate that the
triglycerides tested and glycerol respond to changes in pH in a similar manner.
Culture MlOa shows different responses when methyl acetate and triacetin are
used as substrates and this, therefore, is evidence that the same enzyme system
does not attack triacetin and methyl acetate. The results obtained with culture
P70 are not so clear-cut in this respect.
Downloaded from http://jb.asm.org/ on December 26, 2014 by guest
two definite maxima with only two of the cultures. As in the case of the methyl
esters, culture B4 was able to utilize these substrates only to a very limited extent.
The inhibition by M/6 phosphate buffer is again demonstrated on this type of
substrate with culture P78.
Influence of pH on the oxidation of fatty acid esters. The effect of pH on the
course of various enzyme reactions is well recognized. Esters of saturated fatty
acids were used as substrates. Sodium salts are unsatisfactory because some are
converted to the free acid form at the pH levels used in these experiments.
The results of these pH studies, using acetic and lauric esters, appear in table
7. With methyl acetate as a substrate and in the presence of a phosphate buffer,
cultures B4 and P70 showed optima in the range of pH 7.4 to 7.8, whereas Ml0a
exhibited an optimum at pH 5.8 or below. The data on P78 demonstrate one
optimum at or belowppH 5.8 and another from pH 7.0 to pH 8.0. On ethyl laurate,
6;54
J. J.
JEZESKI,
H.
0. HALVORSON, AND H. MACY
[Vol. 59
Experiments with unsaturated substrates. The oxygen consumption rates in the
presence of methyl esters of stearic, oleic, and linoleic acids were compared to
determine whether the degree of unsaturation would affect the rate of oxidation.
Table 9 presents these results and some of the information is in harmony with
TABLE 9
The influence of the degree of saturation of fatty acid esters on oxygen
consumption by several bacteria
(DAYS)
CU
AGE
Methyl
Methyl linoleate
Methyl oleate
Flask buffer conc.
MlOa
P70
P78
B4
5
3
6
1
3
1
x/6
x/48
M/6
x/48
40
61
78
48
62
-7
24
48
65
89
168
180
99
18
100
130
45
66
46
-5
15
128
118
249
254
122
77
10
_x/6
131*
-23t
154
94
60
83
1
x/48
156*
-14t
315
306
154
170
2
These data are corrected for endogenous respiration and autoxidation of the unsaturated esters.
Time, 90 min; buffer (Clark's phosphate), pH 7.8; substrates, 0.017 M, flask conc.
* Peroxide value, methyl linoleate = 4.0 to 5.0 milliequivalents per gram.
t Peroxide value, methyl linoleate = 19.0 to 20.0 milliequivalents per gram.
TABLE 10
The influence of the pH of the buffer on oxygen consumption of culture P78 in
the presence of several fatty acid esters
MICROLITERS OF OXYGEN CONSUMD AT
Methyl stearate
Methyl linoleate
pH 6.1
pH 6.5
pH 7.0
pH 7.4
pH 8.0
119
89*
141
133
144
109
142
131
131
89
141
143
101
195
143
95*
Time, 90 min; buffer (Clark's phosphate), M/48, flask cone.; substrates, 0.033 m, flask
cone.
* All similar values corrected for
endogenous respiration.
data described earlier. Culture B4 produced only a small amount of oxidation on
stearate and oleate, and linoleate was untouched. According to these data the
oxidative ability of this organism toward the Cis esters decreased as the amount
of unsaturation increased. The rate of oxidation of these esters by culture P70
increased directly with the amount of unsaturation in the presence of M/48
Downloaded from http://jb.asm.org/ on December 26, 2014 by guest
ICRlOITElRS OF OXYGEN CONSUMED IN MME PRESENCE OF
stearate
1950]
ACTION OF MICROORGANISMS ON FATS
655
DISCUSSION
The results obtained in the experiments on buffer type comparisons may be
compatible with those obtained on fat-oxidizing enzymes of animal origin,
namely, that inorganic phosphate was required if adenylic acid, and not adenosine
triphosphate (ATP), was present. The results observed in the experiments could
be explained on the basis that sufficient ATP was present in the cells so that
little difference was observed between the bicarbonate and the phosphate buffers, especially since the strength of the phosphate buffer was relatively weak.
However, stimulation of oxidation by increased phosphate buffer concentrations
in the case of two cultures does indicate that inorganic phosphate may be involved in the process. The inhibition by borate buffer observed with the four
cultures studied is likewise observed in the case of an enzyme from rat liver
capable of oxidizing long-chain fatty acids.
The inhibition of oxygen consumption by M/6 phosphate buffer in the presence
of natural and pure triglycerides and saturated fatty acid esters and salts, as
well as unsaturated esters, occurred with the two strongly lipolytic cultures, B4
Downloaded from http://jb.asm.org/ on December 26, 2014 by guest
buffer. A similar response was shown by, culture Ml0a. P78 does not show the
same type of results since unsaturation does not cause increased oxygen consumption with young cells.
A comparison of the results obtained with M/6 and M/48 buffer indicates that
the inhibition of oxidation of unsaturated substrates also takes place just as with
other substrates tested in the case of two cultures, P70 and P78. It is of interest
to note that with P78, though there is complete inhibition of oxygen uptake in the
presence of stearate and oleate with M/6 buffer, the linoleate undergoes considerable oxidation. It should be noted that the actual decrease in oxygen utilized in
the presence of linoleate due to the use of the M/6 buffer is about equal to that decrease observed in the case of complete inhibition with stearate and oleate as a
substrate. These results are, therefore, indicative of the presence of an enzyme
capable of oxidizing linoleate (probably at the double bonds) that was relatively uninbibited by the higher concentration of phosphate.
The peculiar results obtained with Ml0a on the linoleate substrate cannot be
explained readily except on the basis of inhibition due to autoxidation products
in the substrate. It should be observed that the peroxide values on the substrate
increased from 4.0 to 5.0 up to 19.0 to 20.0 milliequivalents per gram in the
2-day interval. Several later experiments have shown that the inhibition takes
place only in the presence of substrate that showed evidence of autoxidation.
Further evidence that P78 may contain an enzyme specific for linoleate was
gathered in studies on pH optima. Table 10 shows the response of this culture
to various pH levels in the presence of stearate and linoleate esters. Methyl
stearate appears to be oxidized at maximum rates over a broad range of pH 7.0
to 8.0, and the same is true for ethyl laurate. On the other hand, linoleate shows
a sharp increase in oxygen consumption above pH 7.4, and the optimum appears
to be at or above pH 8.0 in the presence of phosphate buffer.
656
J. J.
JEZESKI, H. 0.
HALVORSON,
AND H.
MACY
[vol. 59
Downloaded from http://jb.asm.org/ on December 26, 2014 by guest
and P78. Just what role a lipase plays in this phenomenon is questionable;
nevertheless, the well-known inhibitory effect of phosphate buffer on the action
of lipases is not to be overlooked in this regard. A better explanation may lie
in the effect of the phosphate on some component of the respiratory system of
the susceptible organism, for example, the effect of the buffer salts on certain
trace elements (Mn++ and Mg++) generally supposed to be required for fatty
acid oxidation. This seems quite logical since the inhibition may take place in the
presence of a variety of fatty substrates and glycerol.
However, the use of the sodium salts or soaps of the fatty acids presented
some problems since the method of preparation might influence their properties
as substrates. The pH adjustments were particularly troublesome with those
longer-chain soaps which formed colloidal systems at the pH values used in
these experiments. It is well known that the physical state of the micelles
is influenced by the method of producing and neutralizing the soaps. The charge
on the micelle is likewise influenced by the pH of the system and the direction
in which the pH is shifted. The inconsistencies, when vigorous oxidation occurred with one member of the series and inhibition of respiration took place
with the next homologue, are probably due to these inherent complexities of the
substrate system.
In spite of these difficulties with the sodium salts, the data do show that
the cultures studied differ in their behavior toward the various substrates used.
Although the four cultures did not oxidize the substrates at equal rates, they exhibited similar relative behavior toward the various homologues of the series.
The data obtained on the esters of the saturated fatty acids indicate that at
least two different enzymes are responsible for the oxidation of the members of
the series. This statement is supported by data obtained in studies of substrate
specificity and pH optima. Even though the pH optima recorded for the various
cultures were in general not sharply defined, it seems logical to assume that they
represent true optima, especially since, in all instances, the values corrected for
endogenous respiration present the same picture.
When unsaturated substrates were used, several cultures exhibited greater
oxidative activity as the amount of unsaturation increased. The data obtained on
culture P78 indicate the possibility of the existence of an enzyme acting on the
linoleate that is different than the one attacking stearate, both from the results
obtained on pH optima and the inhibition produced by M/6 phosphate buffer.
The degree of inhibition produced by phosphate buffer on stearate and oleate as
compared with linoleate are indicative of the presence not only of an enzyme
capable of ,8-oxidation according to the classical scheme, but also an enzyme
specific for the two double bonds, since it is only in this respect that the substrates differ. Such an enzyme has been demonstrated in several natural materials.
The peculiar inhibition of culture MlOa due to autoxidized linoleate would
also indicate that an enzyme specific for linoleate is present, especially since
mixing oxidized linoleate with stearate does not produce competitive inhibition.
1950]
ACTION OF MICROORGANISMS ON FATS
657
SUMMARY
REFERENCES
BARRON, E. S. G., AND FRIEDEMANN, T. E. 1941 Studies on biological oxidations. XIV.
Oxidations by microorganisms which do not ferment glucose. J. Biol. Chem., 137,
593-610.
CAST1ELL, C. H., AND GARRARD, E. H. 1941 The action of microorganisms on fat. III.
Oxidation and hydrolysis of triolein by pure cultures of bacteria. Can. J. Research,
19C, 106-110.
HOROWITZ-WLASSOVA, L. M., AND LIVSCHITZ, M. J. 1935 Zur Frage der Wirkung der
Mikroben auf Fette. Zentr. Bakt. Parasitenk., II, 92, 424-435.
JENSEN, L. B., AND GRETTIE, D. P. 1933 Action of microorganisms on fats. Oil and
Soap, 10, 23-32.
JENSEN, L. B., AND GRETTIE, D. P. 1937 Action of microorganisms on fats. Food Research, 2, 97-120.
LANDY, M., AND DICKEN, D. M. 1942 A microbiological assay method for six B vitamins
using Lactobacillus casei and a medium of essentially known composition. J. Lab.
Clin. Med., 27, 1086-1092.
Downloaded from http://jb.asm.org/ on December 26, 2014 by guest
Four bacterial cultures of differing morphological and physiological characteristics were selected for detailed study from 30 cultures capable of showing
increased rates of oxygen consumption in the presence of fat.
These organisms demonstrated similar rates of oxygen uptake in the presence
of bicarbonate and phosphate buffers (M/60, final concentration) but were definitely inhibited in the presence of borate buffer. Increased concentrations of phosphate buffer produced greater rates of oxygen consumption with coconut oil as
a substrate in the case of two cultures. On the other hand, the two strongly
lipolytic cultures were inhibited in their oxygen consumption by the increased
concentrations of phosphate buffer (up to M/6) in the presence of all types of
lipid substrates tested.
Coconut oil was oxidized by the four cultures at a greater rate than either
corn oil or butterfat, whereas four pure triglycerides were attacked at varying
rates. Good evidence has been obtained that oxygen consumption in the presence
of fats may be due to the utilization of glycerol resulting from the action of
lipase. This applies particularly to the Micrococcus culture (B4).
The data indicate that at least two enzymes are involved in the oxidation of
compounds of the saturated fatty acid series by the individual culture; one is
specific for the short-chain and one for the long-chain fatty acids.
The pH optima for the oxidation of the saturated fatty acid substrates by
Mycobacterium phli were in the vicinity of pH 7.8 for ethyl laurate and about
pH 5.8 for methyl acetate. When triacetin was used as a substrate, the optimum
was in the range of pH 7.6 to 7.9. The other cultures showed optima in the range
of pH 7.4 to 7.9 with most of the substrates used in these pH studies.
The presence of an enzyme specific for methyl linoleate is suggested by the
data obtained on a Pseudomonas culture (P78). There is some evidence that
Mycobacterium phlei (MlOa) may posses a similar type of enzyme.
658
J. J.
JEZESKI, H. 0. HALVORSON, AND H. MACY
[vol. 59
Downloaded from http://jb.asm.org/ on December 26, 2014 by guest
LEHNINGER, A. L. 1945a The relationship of the adenosine polyphosphates to fatty acid
oxidation in homogenized liver preparations. J. Biol. Chem., 157, 363-381.
LEHNINGER, A. L. 1945b On the activation of fatty acid oxidation. J. Biol. Chem.,
161, 437-451.
MAZZA, F. P., AND CIMMINO, A. 1933 Sull'attivita deidrogenasica del B. coli communis
sugli acidi grassi superiori. Boll. soc. ital. biol. sper., 8, 531-534.
MUNDT, J. O., AND FABIAN, F. W. 1944 The bacterial oxidation of corn oil. J. Bact.,
48, 1-11.
NIVEN, C. F., JR., EVANS, J. B., AND WHITE, J. C. 1945 Oxidation of butyric acid by
streptococci. J. Bact., 49, 105.
PIGUIWSKI, G., AND CHARIK, N. 1929 Zersetzung des Olivenols, unter dem Einfluss
der vitalen TAtigkeit, einiger Mikroorganismen: Umwandlung von Oleinsiure in Ketostearinsiure. Biochem.. Z., 200, 201-210.
QUASTEL, J. B., AND WHETHAM, M. D. 1925 Dehydrogenations produced by resting bacteria. I. Biochem. J., 19, 520-531.
SINGER, T. P., AND BARRON, E. S. G. 1945 Studies on biological oxidations. XX. Sulfhydryl enzymes in fat and protein metabolism. J. Biol. Chem., 157, 241-253.
STARK, C. N., AND SCHEIB, B. J. 1936 A study of fat-splitting and casein-digesting bacteria isolated from butter. J. Dairy Sci., 19, 191-213.
UM[BREIT, W. W., BURRIS, R. H., AND STAUFFER, J. F. 1945 Manometric techniques and
related methods for the study of tissue metabolism. Burgess Publishing Co., Minneapolis.
You might also like
- Pyruvate and Fatty Acid MetabolismFrom EverandPyruvate and Fatty Acid MetabolismRating: 1.5 out of 5 stars1.5/5 (2)
- Degradation Kraft Indulin Lignin by Viridosporus Streptomyces BadiusDocument7 pagesDegradation Kraft Indulin Lignin by Viridosporus Streptomyces BadiusMuhammad UmerNo ratings yet
- Studies On TO Toxins of Clostridium Botulinum A Simplified ProcedureDocument6 pagesStudies On TO Toxins of Clostridium Botulinum A Simplified ProcedureAustinNo ratings yet
- Biochem - Pityrosporum OvaleDocument7 pagesBiochem - Pityrosporum OvaleJuliana SoaresNo ratings yet
- Effect of Food Preservatives On Growth and Aflatoxin Production of Us Flavus in Liquid MediumDocument4 pagesEffect of Food Preservatives On Growth and Aflatoxin Production of Us Flavus in Liquid MediumPretty KungkingNo ratings yet
- J. Bacteriol.-1957-Duff-597-601 PDFDocument5 pagesJ. Bacteriol.-1957-Duff-597-601 PDFnein mein100% (1)
- Biotin 2Document12 pagesBiotin 2Lars Ben HayahayNo ratings yet
- 21-Metabolite+of+P +ostDocument6 pages21-Metabolite+of+P +ostAndrea GNo ratings yet
- Leyer & Johnson (1997)Document7 pagesLeyer & Johnson (1997)Kharisma N. PuspitasariNo ratings yet
- A02910106 With Cover Page v2Document7 pagesA02910106 With Cover Page v2Gaurav ChauhanNo ratings yet
- Schaefer1965 Effect of Oleic Acid On Growth and Cell Structure of MycobacteriaDocument10 pagesSchaefer1965 Effect of Oleic Acid On Growth and Cell Structure of MycobacteriaNathan IbaleNo ratings yet
- Fermentação de Glicose Por ChlorellaDocument8 pagesFermentação de Glicose Por ChlorellapaulavonNo ratings yet
- Sputum Digestion and Decontamination With N-acetyl-L-cysteine-sodium Hydroxide For Culture of MycobacteriaDocument5 pagesSputum Digestion and Decontamination With N-acetyl-L-cysteine-sodium Hydroxide For Culture of MycobacteriaMax Ruiz NizamaNo ratings yet
- Agua de Coco AaaDocument14 pagesAgua de Coco AaaMaría Del Mar LondoñoNo ratings yet
- 1960 - Amino Acid Decarboxylases in A PseudomonaDocument7 pages1960 - Amino Acid Decarboxylases in A PseudomonaGiancarlo GHNo ratings yet
- The Use of Solid Media For Detection of Enzyme Production by FungiDocument12 pagesThe Use of Solid Media For Detection of Enzyme Production by FungiRenan CamposNo ratings yet
- Acido LacticoDocument9 pagesAcido LacticoJuan Victor ChampeNo ratings yet
- The Effects of Nature of Substrate On TH PDFDocument20 pagesThe Effects of Nature of Substrate On TH PDF6A(24) Marsh WongNo ratings yet
- 12 59 Lipolytic Enzymesarticle27Document8 pages12 59 Lipolytic Enzymesarticle27Furqoni Nurul UmmahNo ratings yet
- Lipase AssayDocument6 pagesLipase AssayMuhammad Zaki ArraziNo ratings yet
- Yeast Aeration FatDocument8 pagesYeast Aeration FatffwwfNo ratings yet
- Sarrafzadeh Et Al. - 2005 - Dielectric Monitoring of Growth and Sporulation of Bacillus ThuringiensisDocument7 pagesSarrafzadeh Et Al. - 2005 - Dielectric Monitoring of Growth and Sporulation of Bacillus ThuringiensisanjaliboseNo ratings yet
- Regulation of Succinate Dehydrogenase in E-ColiDocument7 pagesRegulation of Succinate Dehydrogenase in E-ColiRavi KumarNo ratings yet
- Enhanced Cellulase Production in Fed-Batch Solid State Fermentation of SL-1Document4 pagesEnhanced Cellulase Production in Fed-Batch Solid State Fermentation of SL-1Mella Rosa SebiferaNo ratings yet
- The Acid Tolerance Response of Bacillus Cereus ATCC14579 Is Dependent On Culture PH, Growth Rate and Intracellular PHDocument11 pagesThe Acid Tolerance Response of Bacillus Cereus ATCC14579 Is Dependent On Culture PH, Growth Rate and Intracellular PHخديجة بيوNo ratings yet
- Lipopeptida Bacillus SPDocument5 pagesLipopeptida Bacillus SPDikaputriedria NingtyasNo ratings yet
- Hildegarde Esther Allen: City of Medical AND Division OF OFDocument7 pagesHildegarde Esther Allen: City of Medical AND Division OF OFJulien Patrick CebrianNo ratings yet
- Anthony, 1963Document6 pagesAnthony, 1963manda_505No ratings yet
- Appl. Microbiol.-1971-Kim-581-7Document7 pagesAppl. Microbiol.-1971-Kim-581-7gmanju207No ratings yet
- Research Article: Influence of Elicitation and Germination Conditions On Biological Activity of Wheat SproutsDocument9 pagesResearch Article: Influence of Elicitation and Germination Conditions On Biological Activity of Wheat Sproutsmonica lestaryNo ratings yet
- Bacilluslicheniformis 599Document7 pagesBacilluslicheniformis 599cbb.chintan7014No ratings yet
- The Differentiation Basis Arginine Metabolism Other Gram-Negative Bacteria On TheDocument16 pagesThe Differentiation Basis Arginine Metabolism Other Gram-Negative Bacteria On TheFrancisca Beltrán GuzmánNo ratings yet
- JB 82 4 582-588 1961Document7 pagesJB 82 4 582-588 1961laasyagudiNo ratings yet
- Electronic Journal of Biotechnology: Ying Yu, Xiaoyu Zhou, Sheng Wu, Tiantian Wei, Long YuDocument6 pagesElectronic Journal of Biotechnology: Ying Yu, Xiaoyu Zhou, Sheng Wu, Tiantian Wei, Long YuSadieNo ratings yet
- Expt 11 Lab ReportDocument11 pagesExpt 11 Lab ReportGracechel PormildaNo ratings yet
- Effect of Water ActivityDocument2 pagesEffect of Water ActivityMariel GeleraNo ratings yet
- From The Biological Division of The Department of Medicine, Johns Hopkins University Medical School, BaltimoreDocument13 pagesFrom The Biological Division of The Department of Medicine, Johns Hopkins University Medical School, BaltimoreEfa BonitaNo ratings yet
- Lee Et Al 1998Document3 pagesLee Et Al 1998rinifiahNo ratings yet
- History: Purpose The Oxidative-Fermentative Test Is Used To Determine If Gram-NegativeDocument3 pagesHistory: Purpose The Oxidative-Fermentative Test Is Used To Determine If Gram-NegativeSujit ShandilyaNo ratings yet
- Journal of Bacteriology 1959 Widra 664.fullDocument7 pagesJournal of Bacteriology 1959 Widra 664.fullAparna VRNo ratings yet
- Batch and Fed-Batch Production of Butyric Acid by Clostridium Butyricum ZJUCBDocument5 pagesBatch and Fed-Batch Production of Butyric Acid by Clostridium Butyricum ZJUCBapi-3743140No ratings yet
- 2685 FullDocument8 pages2685 FullAndréRochaNo ratings yet
- Optimization of A Fed-Batch Fermentation Process For Production of Bleomycin by Streptomyces Mobaraensis ATCC 15003Document6 pagesOptimization of A Fed-Batch Fermentation Process For Production of Bleomycin by Streptomyces Mobaraensis ATCC 15003jmrozo3No ratings yet
- Action of Synthetic Detergents On TheDocument23 pagesAction of Synthetic Detergents On TheTalita ColomeuNo ratings yet
- Isolation and Purification of Peroxidase From Shoots of OF TOMATODocument9 pagesIsolation and Purification of Peroxidase From Shoots of OF TOMATOPooja WalkeNo ratings yet
- Abreu 2005Document6 pagesAbreu 2005claraNo ratings yet
- 4w100002599 PDFDocument8 pages4w100002599 PDFPrakashNo ratings yet
- InvitrosolubilizationDocument6 pagesInvitrosolubilizationmuhammadrafiqyNo ratings yet
- Physicochemical Studies On SchizophyllumDocument5 pagesPhysicochemical Studies On SchizophyllumiraNo ratings yet
- Embriogenesis SomaticaDocument5 pagesEmbriogenesis SomaticaKarinNo ratings yet
- Evaluation of Support Matrices For Immobilization of Anaerobic Consortia For e Cient Carbon Cycling in Waste RegenerationDocument10 pagesEvaluation of Support Matrices For Immobilization of Anaerobic Consortia For e Cient Carbon Cycling in Waste RegenerationGregorius BudiantoNo ratings yet
- Industrial Microbiology Lab 1 (BTC4205)Document23 pagesIndustrial Microbiology Lab 1 (BTC4205)Jerry CaldwellNo ratings yet
- Preparation and Characterization of Doxorubicin-Containing Liposomes: I. Influence of Liposome Charge and PH of Hydration Medium On Loading Capacity and Particle SizeDocument14 pagesPreparation and Characterization of Doxorubicin-Containing Liposomes: I. Influence of Liposome Charge and PH of Hydration Medium On Loading Capacity and Particle SizeSanelaNo ratings yet
- Murashige SkoogDocument26 pagesMurashige SkoogArturo MunozNo ratings yet
- Group Work 1Document8 pagesGroup Work 1Tlotliso MphomelaNo ratings yet
- Vol. 7I Human Seminal Acid Phosphatase 233: TransportDocument10 pagesVol. 7I Human Seminal Acid Phosphatase 233: TransportAlishba KaiserNo ratings yet
- Microorganisms 08 00286Document22 pagesMicroorganisms 08 00286Bagus OktiNo ratings yet
- Baltagul Mihail SadoveanuDocument6 pagesBaltagul Mihail SadoveanuMircea Ciutaru0% (2)
- Diversity of Bacterial Strains Degrading Hexadecane in Relation To The Mode of Substrate UptakeDocument8 pagesDiversity of Bacterial Strains Degrading Hexadecane in Relation To The Mode of Substrate Uptakesaiful2016No ratings yet
- A As in AreDocument1 pageA As in AreSwati2013No ratings yet
- Few Words by Lala Lajpat Rai Ji : IndiaDocument2 pagesFew Words by Lala Lajpat Rai Ji : IndiaSwati2013No ratings yet
- 3.2.2015 Functions of LipidsDocument6 pages3.2.2015 Functions of LipidsSwati2013No ratings yet
- A Role For Dicer in Immune Regulation: ArticleDocument9 pagesA Role For Dicer in Immune Regulation: ArticleSwati2013No ratings yet
- Joining The Start With The ExtremeDocument3 pagesJoining The Start With The ExtremeSwati2013No ratings yet
- Nano BiotechnologyDocument2 pagesNano BiotechnologySwati2013No ratings yet
- Rep Sequences: DiscoveryDocument2 pagesRep Sequences: DiscoverySwati2013No ratings yet
- Pour Plate Technique: For Bacterial EnumerationDocument2 pagesPour Plate Technique: For Bacterial EnumerationSwati2013No ratings yet
- Chromatin Immunoprecipitation (Chip) : Michael F. Carey, Craig L. Peterson and Stephen T. SmaleDocument9 pagesChromatin Immunoprecipitation (Chip) : Michael F. Carey, Craig L. Peterson and Stephen T. SmaleSwati2013No ratings yet
- Isolating "Uncultivable" Microorganisms in Pure Culture in A Simulated Natural EnvironmentDocument3 pagesIsolating "Uncultivable" Microorganisms in Pure Culture in A Simulated Natural EnvironmentSwati2013No ratings yet
- A Teacher For All Seasons PoemDocument2 pagesA Teacher For All Seasons PoemSwati2013No ratings yet
- CadDocument8 pagesCadRamesh Babu GarlapatiNo ratings yet
- Ecteinascidin 743 (080414-TKGP) T. Fukuyama: Activity Key ReactionsDocument3 pagesEcteinascidin 743 (080414-TKGP) T. Fukuyama: Activity Key ReactionsPercival GalahadNo ratings yet
- Lisca - Lingerie Catalog II Autumn Winter 2013Document76 pagesLisca - Lingerie Catalog II Autumn Winter 2013OvidiuNo ratings yet
- Protein ChemistryDocument3 pagesProtein ChemistryAriane Manalo CerezoNo ratings yet
- Me Sci 10 q3 1301 PsDocument27 pagesMe Sci 10 q3 1301 PsCassy Leighz BagonocNo ratings yet
- Dyslipidemia 2021Document86 pagesDyslipidemia 2021Rania ThiniNo ratings yet
- Perbekalan Farmasi RekapDocument556 pagesPerbekalan Farmasi RekapChelseaNo ratings yet
- General and Specific Tests For CarbohydratesDocument13 pagesGeneral and Specific Tests For CarbohydratesKai Chen50% (2)
- Regulation of Cellular Respiration (Article) - Khan AcademyDocument12 pagesRegulation of Cellular Respiration (Article) - Khan Academydeepali_nih9585No ratings yet
- DEC14Document24 pagesDEC14L JNo ratings yet
- Codex Stan 32-1981, Rev.1-1989 Codex Standard For Margarine (5p)Document5 pagesCodex Stan 32-1981, Rev.1-1989 Codex Standard For Margarine (5p)Mark KwanNo ratings yet
- Connectivity and Binding-Site Recognition ApplicationsDocument12 pagesConnectivity and Binding-Site Recognition Applicationsfiw ahimNo ratings yet
- Molecular Weight CalculatorDocument9 pagesMolecular Weight Calculatornil82No ratings yet
- Lyphochek Assayed Chemistry Control Levels 1 and 2: Revision Date 2022-05-16 Indicates Revised InformationDocument23 pagesLyphochek Assayed Chemistry Control Levels 1 and 2: Revision Date 2022-05-16 Indicates Revised InformationAjish joNo ratings yet
- 039 Arctic Sea ENG 9-15-10 PDFDocument1 page039 Arctic Sea ENG 9-15-10 PDFManoj KumarNo ratings yet
- Chemical Basis of LifeDocument5 pagesChemical Basis of LifeCaithlyn KirthleyNo ratings yet
- UGEB2363-1718-week 5 To 6Document70 pagesUGEB2363-1718-week 5 To 6Gladys Gladys MakNo ratings yet
- Top Antihypertensive Drugs Generic-Brand Names PDFDocument1 pageTop Antihypertensive Drugs Generic-Brand Names PDFvidbala0% (1)
- Summary Ubat 211 TambahanDocument31 pagesSummary Ubat 211 Tambahancks09No ratings yet
- Cognis College: Fatty AcidsDocument22 pagesCognis College: Fatty AcidsRidhuan Dion100% (3)
- Biochemistry Exam 1 ReviewDocument37 pagesBiochemistry Exam 1 ReviewThomas B.100% (1)
- CPP (Biomolecules) : Part - I: Subjective QuestionsDocument15 pagesCPP (Biomolecules) : Part - I: Subjective QuestionsFalgun SoniNo ratings yet
- True or FalseDocument4 pagesTrue or Falsetaya guyNo ratings yet
- 1 The Central Dogma of Molecular BiologyDocument6 pages1 The Central Dogma of Molecular Biologydeladestianiaji2490100% (1)
- TB 8 PDFDocument2 pagesTB 8 PDFCatalina AncaNo ratings yet
- Organik CompoundsDocument12 pagesOrganik CompoundsInas MellanisaNo ratings yet
- Ans311 Notes - Sept. 2020Document141 pagesAns311 Notes - Sept. 2020Joy.B mwanzaNo ratings yet
- Isi FornasDocument239 pagesIsi FornasVrizskiNo ratings yet
- Structure of Organic CompoundsDocument27 pagesStructure of Organic CompoundsBetty Weiss100% (1)
- SSTDocument70 pagesSSTnaelarizqi0% (1)