Professional Documents
Culture Documents
TB341
Uploaded by
reveoCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
TB341
Uploaded by
reveoCopyright:
Available Formats
User Protocol TB341 Rev.
D 0111JN
Page 1 of 9
KOD Hot Start DNA Polymerase
Table of Contents
About the Kits ......................................................................................................... 2
Description
Components
Storage
2
3
3
KOD Hot Start DNA Polymerase Protocol ............................................................. 4
Standard reaction setup
Cycling conditions
4
4
Additional Guidelines ............................................................................................. 5
Primers
Template DNA
Reaction components
Extension temperature and time
Two-step PCR
Optimization
5
5
6
6
6
6
Troubleshooting ..................................................................................................... 7
Application references ........................................................................................... 7
References ............................................................................................................. 8
Appendix ................................................................................................................ 8
Example amplifications
2011 EMD Chemicals, Inc., an affiliate of Merck KGaA, Darmstadt, Germany. All rights reserved. Perfectly Blunt and Novagen are registered
trademarks of Merck KGaA, Darmstadt, Germany. TWEEN is a registered trademark of ICI Americas, Inc. PfuTurbo and PfuUltra are registered
trademarks of Stratagene. KOD Polymerases are manufactured by TOYOBO and distributed by EMD Chemicals, Inc. KOD XL DNA Polymerase is licensed
under U.S. Patent No. 5,436,149 owned by Takara Shuzo, Co., Ltd.
Use of this product is covered by one or more of the following US patents and corresponding patent claims outside the US: 5,789,224, 5,618,711, 6,127,155
and claims outside the US corresponding to expired US Patent 5,079,352. The purchase of this product includes a limited, non-transferable immunity from
suit under the foregoing patents claims for using only this amount of product for the purchaser's own internal research. No right under any other patent claim,
and no right to perform commercial services of any kind, including without limitation reporting the results of purchaser's activities for a fee or other comical
consideration, is conveyed expressly, by implication, or by estoppel. This product is for research use only. Diagnostic uses under Roche patents require a
separate license from Roche. Further information on purchasing licenses may be obtained by contacting the Direction of Licensing, Applied Biosystems, 850
Lincoln Centre Drive, Foster City, California 94404, USA.
USA and Canada
Tel (800) 628-8470
bioscienceshelp@
emdchemicals.com
Europe
France
Freephone
0800 126 461
Germany
Freecall
0800 100 3496
Ireland
Toll Free
1800 409 445
All Other Countries
United Kingdom
Freephone
0800 622 935
All other
Contact Your Local Distributor
European Countries
www.merck4biosciences.com
bioscienceshelp@
+44 115 943 0840
emdchemicals.com
techservice@merckbio.eu
www.merck4biosciences.com
FOR RESEARCH USE ONLY. NOT FOR HUMAN OR DIAGNOSTIC USE.
Cat. No.
User Protocol TB341 Rev. D 0111JN
Page
2 of 9
Page
About the Kits
KOD Hot Start DNA Polymerase
20 U
200 U
1000 U
71086-5
71086-3
71086-4
Description
KOD Hot Start DNA Polymerase is a premixed complex of the high fidelity KOD DNA Polymerase and two monoclonal
antibodies that inhibit the DNA polymerase and 3'5' exonuclease activities at ambient temperatures (1). KOD Hot Start
combines the high fidelity, fast extension speed, and outstanding processivity of KOD with the high specificity of an
antibody-mediated hot start. Non-specific amplification is reduced because mispriming events during reaction set up and
the initial temperature increase are avoided. In addition, primer degradation during setup at ambient temperature due to
exonuclease activity is effectively inhibited. This enzyme quickly and accurately amplifies genomic and phage/plasmid
DNA targets up to 12 and 20 kbp, respectively. GC-rich targets are also efficiently amplified. KOD Hot Start DNA
Polymerase produces blunt-ended DNA products that are suitable for cloning with the Novagen Perfectly Blunt and LIC
Vector Kits and is compatible with site-directed mutagenesis protocols.
Unit definition: One unit is defined as the amount of enzyme that will catalyze the incorporation of 10 nmol of dNTP
into acid insoluble form in 30 minutes at 75C in a reaction containing 20 mM Tris-HCl (pH 7.5 at 25C), 8 mM MgCl2,
7.5 mM DTT, 50 g/ml BSA, 150 M each of dATP, dCTP, dGTP, dTTP (a mix of unlabeled and [3H]-dTTP) and
150 g/ml activated calf thymus DNA.
Polymerase fidelity comparison
Mutation frequency comparison: KOD Hot Start, PfuTurbo, PfuUltra, and Taq
The fidelity of replication was measured as the mutation frequency in PCR products using a modified rpsL+ fidelity assay
(2, 3).
Polymerase rate comparison
Enzyme
KOD
DNA Polymerase
Pfu
DNA Polymerase
Taq
DNA Polymerase
Species
Thermocccus
kodakaraensis
Pyrococcus furiosus
Thermus aquaticus YT-1
Elongation rate (bases/second)
106138
25
61
Processivity* (nucleotide bases)
> 300
< 20
not determined
* Processivity is defined as the number of nucleotides that can be extended in one catalytic reaction by one DNA polymerase molecule.
USA and Canada
Tel (800) 628-8470
bioscienceshelp@
emdchemicals.com
Germany
Tel 0800 100 3496
techservice@merckbiosciences.de
United Kingdom and Ireland
UK Freephone 0800 622935
Ireland Toll Free 1800 409445
customer.service@merckbiosciences.co.uk
All Other Countries
www.merck4biosciences.com
bioscienceshelp@
emdchemicals.com
Cat. No.
User Protocol TB341 Rev. D 0111JN
Page
3 of 9
Page
Components
20 U or 200 U or 5 200 U
1.2 ml or 5 1.2 ml
1 ml or 5 1 ml
1 ml or 5 1 ml
KOD Hot Start DNA Polymerase (1 U/l in 50 mM Tris-HCl, 1mM DTT,
0.1 mM EDTA, 50% glycerol, 0.001% Nonidet P-40, 0.001% Tween 20,
pH 8.0)
10X PCR Buffer for KOD Hot Start DNA Polymerase
25 mM MgSO4
dNTPs (2 mM each)
Storage
Store all components at 20C.
USA and Canada
Tel (800) 628-8470
bioscienceshelp@
emdchemicals.com
Germany
Tel 0800 100 3496
techservice@merckbiosciences.de
United Kingdom and Ireland
UK Freephone 0800 622935
Ireland Toll Free 1800 409445
customer.service@merckbiosciences.co.uk
All Other Countries
www.merck4biosciences.com
bioscienceshelp@
emdchemicals.com
Cat. No.
User Protocol TB341 Rev. D 0111JN
Page
4 of 9
Page
KOD Hot Start DNA Polymerase Protocol
KOD Hot Start DNA Polymerase and buffer are a unique PCR system. The following procedure is designed for use
with the components provided in the KOD Hot Start DNA polymerase kit. Using reaction components or protocols
designed for any other DNA polymerase may result in poor amplification. Reaction conditions listed below will
provide satisfactory amplification for most primer/template combinations. Guidelines and troubleshooting sections
provide details for optimizing reaction conditions.
Examples of amplification from human genomic DNA and plasmid DNA can be found in the Appendix on page 8.
Standard reaction setup
Component
Volume
Final Concentration
10X Buffer for KOD Hot Start DNA Polymerase
5 l
1X
25 mM MgSO4a
3 l
1.5 mM
dNTPs (2 mM each)
5 l
0.2 mM (each)
PCR Grade Water
X l
Sense (5') Primer (10 M)
1.5 l
0.3 M
Anti-Sense (3') Primer (10 M)
1.5 l
0.3 M
Template DNAb
Y l
KOD Hot Start DNA Polymerase (1 U/l)
1 l
Total reaction volume
50 l
0.02U/l
To optimize for targets greater than 2kb, final Mg2+ concentration may be adjusted to between
1.5 and 2.25 mM.
See Template DNA section on page 5.
Cycling conditions
Temperature and time
The following table allows for primer extension that occurs during temperature ramping between steps.
Target size
< 500 bp
5001000 bp
10003000 bp
> 3000 bp
1. Polymerase activation
Step
95C for 2 min
95C for 2 min
95C for 2 min
95C for 2 min
2. Denature
95C for 20 s
95C for 20 s
95C for 20 s
95C for 20 s
4. Extension
70C for 10 s/kb
70C for 15 s/kb
Repeat steps 24
2040 cycles. For more information see "Cycle number" below
3. Annealing
Lowest Primer TmC for 10 s
70C for 20 s/kb
70C for 25 s/kb
Cycle number
The number of cycles (steps 2 through 4 in the above table) required to generate a PCR product will depend on the
source and amount of starting template in the reaction, as well as the efficiency of the PCR. In general, 2040 cycles will
be adequate for a wide range of templates. It is common to use fewer cycles when amplifying targets from plasmids
(i.e., subcloning) where a high number of copies of template is easily attained, as this reduces the chance of amplifying a
mutation. A higher number of cycles (e.g., 40) may be necessary when amplifying from genomic DNA since the target
sequence will be in low abundance.
USA and Canada
Tel (800) 628-8470
bioscienceshelp@
emdchemicals.com
Germany
Tel 0800 100 3496
techservice@merckbiosciences.de
United Kingdom and Ireland
UK Freephone 0800 622935
Ireland Toll Free 1800 409445
customer.service@merckbiosciences.co.uk
All Other Countries
www.merck4biosciences.com
bioscienceshelp@
emdchemicals.com
Cat. No.
User Protocol TB341 Rev. D 0111JN
Page
5 of 9
Page
Additional Guidelines
Primers
Primer design is critical for successful PCR amplification. Because KOD Hot Start DNA polymerase exhibits strong
3'5' exonuclease activity after thermo activations, primers should be at least 21 bases of 3' end complementary to the
target sequence. G/C content of the primers should be 4060%. Primer melting temperature (Tm) is defined as the
temperature at which one half of the DNA duplex will dissociate to become single stranded. Some primer molecules will
anneal as the temperature approaches the Tm of a primer, as a result PCR amplifications are usually successful over a
range of annealing temperatures. Primer pairs with similar Tm values usually result in better amplifications because
annealing and extension are better synchronized. If melting temperatures of a primer pair differ by more than 5C,
increasing the length of the lower-Tm primer will reduce the difference.
There are several methods for determining the Tm of a primer. The nearest-neighbor method (4) using 50 mM monovalent
salt is one method for Tm prediction. Unlike other methods, the nearest-neighbor method takes into account the primer
sequence and other variables such as salt and DNA concentration. The Tm can also be calculated with the % GC method
(5). The most general method of calculating the Tm is based on the number of adenine (A), thymidine (T), guanidine (G)
or cytosine (C) bases where Tm(C) = 2(NA + NT) + 4(NG + NC.).
Primer Tm values reported by manufacturers may vary by 5 to 10C depending on the calculation method used. In
addition, the exact Tm for a given primer in a reaction may be affected by DNA concentrations (primer and template),
mono and divalent ion concentrations, dNTP concentration, presence of denaturants (e.g., DMSO), and nucleotide
modifications. Therefore, an optimal primer annealing temperature should be determined empirically.
When receiving oligonucleotides from the manufacturer, prepare primer stocks at 100 pmol/l (100 M) in TE and store
them at 20C. To set up KOD reactions, dilute enough of each primer stock 10-fold (10 M) to add 1.5 l per reaction.
Template DNA
The optimal amount of starting template may vary depending on the template quality. In general the suggested amount of
template DNA for amplification is 10 ng phage DNA, 10 ng plasmid DNA, 100 ng genomic DNA, or 2 l of a reverse
transcription reaction. Using too much template in the PCR reaction can result in failed reactions since template
denaturation is concentration dependant. At high concentrations of DNA, denaturation is less efficient.
Plasmid templates
For subcloning, amplify from 10 ng of plasmid template and reduce the number of cycles to 2025.
GC-rich templates
The addition of DMS0 to 210% final concentration may decrease template secondary structure and increase yield. Final
DMSO concentrations of less than 5% v/v have no effect on fidelity (6, 7). The effect of DMSO above 5% v/v on enzyme
fidelity has not yet been determined.
Unpurified templates
Crude cell lysates, PCR products, plaques, and colonies can serve as template for PCR. Limit the volume of unpurified
templates to reduce inhibition of the reaction.
Long target DNA
Amplification of targets longer than 3000 bp can be improved by increasing the concentration of MgSO4. Adjusting the
final MgSO4 concentration from 1.5 to 2.25 mM in 0.25 mM increments should be tried when suboptimal results are
obtained for targets over 3000 bp. Also, the addition of DMSO to 210% v/v final concentration may reduce secondary
structure of the template DNA and increase yield.
USA and Canada
Tel (800) 628-8470
bioscienceshelp@
emdchemicals.com
Germany
Tel 0800 100 3496
techservice@merckbiosciences.de
United Kingdom and Ireland
UK Freephone 0800 622935
Ireland Toll Free 1800 409445
customer.service@merckbiosciences.co.uk
All Other Countries
www.merck4biosciences.com
bioscienceshelp@
emdchemicals.com
Cat. No.
User Protocol TB341 Rev. D 0111JN
Page
6 of 9
Page
Reaction components
High volumes of primer and template DNA suspended in Tris-EDTA (TE) will chelate free Mg2+ in the reaction and may
affect enzyme performance. If the combined volume of primer and template in TE in the reaction exceeds 5 l, then
adjustment of the MgSO4 concentration may be necessary. Increase the MgSO4 in the reaction to compensate for the
EDTA. Each molecule of EDTA will chelate one molecule of Mg2+, so increasing the MgSO4 by 0.25l (0.125mM) for
every 6.3 l of TE will compensate for the Mg2+ chelated by the EDTA.
Extension temperature and time
Extension at 70C is recommended since a good balance of polymerization speed and accuracy is obtained. KOD Hot
Start DNA Polymerase exhibits optimal proof reading activity at 68C and optimal polymerization activity at 74C. If
using an extension temperature near 74C, shortening the extension time 5 s/kbp may give better amplification. When
using an extension temperature near 68 C, increasing the extension time by 5 s/kbp may give better results.
Two-step PCR
In two-step PCR, annealing and extension can be carried out at the same temperature. Primers for two-step cycling
programs should be designed with high Tm values ( > 65C) to ensure proper annealing and extension at the same
temperature. Initially try an annealing/extension temperature equal to the lowest Tm of the primer pair. Since polymerase
speed is slower at 68C, increase the annealing/extension time by 5 s/kbp during two-step cycling.
Optimization
When optimizing PCR reactions, it is best to change only one parameter at a time. The use of DMSO at 5% v/v final
often improves a suboptimal PCR.
USA and Canada
Tel (800) 628-8470
bioscienceshelp@
emdchemicals.com
Germany
Tel 0800 100 3496
techservice@merckbiosciences.de
United Kingdom and Ireland
UK Freephone 0800 622935
Ireland Toll Free 1800 409445
customer.service@merckbiosciences.co.uk
All Other Countries
www.merck4biosciences.com
bioscienceshelp@
emdchemicals.com
Cat. No.
User Protocol TB341 Rev. D 0111JN
Page
7 of 9
Page
Troubleshooting
Symptom
Possible cause
Solution
No PCR product
Extension time is too long
Lower extension time to 15 s/kpb
Too much secondary structure in template DNA
Add DMSO to a final concentration of 510% v/v
PCR primers are not long enough
Annealing temperature is too high
Use primers longer than 21 bases
Lower annealing temperature in 3C decrements
High GC content
Add DMSO to a final concentration of 510% v/v
Low yield
Suboptimal PCR conditions
Increase final MgSO4 concentration in 0.25 mM increments
Long target DNA
Increase final MgSO4 concentration in 0.25 mM increments
Smearing
Too much template DNA
Reduce the amount of template DNA
Smearing below target
size
Extension times are too short
Increase extension time 5 s/kbp
MgSO4 concentration too low
Increase final MgSO4 concentration 0.25 mM increments
Smearing above target
size
Extension times too long
Reduce extension time 5 s/kbp
MgSO4 concentration too high
Decrease final MgSO4 concentration in 0.25 mM increments
Primer dimers
Primers are complementary to each other
Design primers that are not self-complementary or
complementary to each other
Primer concentration is too high
Reduce primer concentration
Annealing temperature too low
Raise annealing temperature
Application references
This section lists selected references for applications with KOD Hot Start DNA Polymerase. Please visit
www.merck4biosciences.com for more information.
Application
Reference
Colony-direct PCR with Grampositive bacteria
Tsuchizaki, N. and Hotta, K. (2003) inNovations 17, 911
Elongation PCR
Gao, X., Yo, P., Keith, A., Ragan, T. J., and Harris, T. K. (2003) Nucleic Acids Res. 31, e143
Gene cloning
Schilling, O., Spath, B., Kostelecky, B., Marchfelder, A., Meyer-Klaucke, W., and Vogel, A. (2005) J.
Biol. Chem. 280, 1785717862
Williams, M. E., Burton, B., Urrutia, A., Shcherbatko, A., Chavez-Noriega, L. E., Cohen, C.J., and
Aiyar, J. (2005) J. Biol. Chem. 280, 12571263
Miyazato, T., Toma, C., Nakasone, N., Yamamoto, K., and Iwanaga, M. (2003) J. Med. Microbiol. 52,
283288
Gene cloning using consensus
shuffling
Binkowski, B. F., Richmond, K. E., Kaysen, J., Sussman, M. R., and Belshaw, P. J. (2005) Nucleic
Acids Res. 33, e55
Multiplex cDNA-PCR
Sagara, N. and Katho, M. (2000) Cancer Res. 60, 59595962
Multiplexed SNP genotyping
Higasa, K. and Hayashi, K., (2002) Nucleic Acids Res. 30, e11
Mutagenesis
Tabuchi, M., Tanaka, N., Nishida-Kitayama, H., Ohno, H., and Kishi, F. (2002) Mol. Biol. Cell 13,
43715387.
Matsumoto, N., Mitsuki, M., Tajima, K., Yokoyama, W. M., and Yamamoto, K. (2001) J. Exp. Med. 193,
147158.
PCR for PCR-Mass spectrometry
based analysis
Benson, L. M., Null, A. P., and Muddiman, D. C. (2003) J. Am. Soc. Mass. Spectrom. 14, 601604.
PCR for sequence analysis
Okamoto, T., Yoshiyama, H., Nakazawa, T., Park, I. D., Chang, M. W., Yanai, H., Okita, K., and Shirai,
M. (2002) J. Antimicrob. Chemother. 50, 849856.
Second strand cDNA synthesis
Hirohashi, Y., Torigoe, T., Maeda, A., Nabeta, Y., Kamiguchi, K., Sato, T., Yoda, J., Ikeda, H., Hirata,
K., Yamanaka, N., and Sato, N. (2002) Clin. Cancer Res. 8, 17311739.
USA and Canada
Tel (800) 628-8470
bioscienceshelp@
emdchemicals.com
Germany
Tel 0800 100 3496
techservice@merckbiosciences.de
United Kingdom and Ireland
UK Freephone 0800 622935
Ireland Toll Free 1800 409445
customer.service@merckbiosciences.co.uk
All Other Countries
www.merck4biosciences.com
bioscienceshelp@
emdchemicals.com
Cat. No.
User Protocol TB341 Rev. D 0111JN
Page
8 of 9
Page
References
1. Mizuguchi, H., Nakatsuji, M., Fujiwara, S., Takagi, M. and Imanaka, T. (1999) J. Biochem (Tokyo) 126, 762768.
2. Kitabayashi, M., Nishiya, Y., Esaka, M., Itakura, M., and Imanaka, T. (2002) Biosci. Biotechnol. Biochem. 66,
21942200.
3. Fujii, S., Akiyama, M., Aoki, K., Sugaya, Y., Higuchi, K., Hiraoka, M., Miki, Y., Saitoh, N., Yoshiyama, K., Ihara, K.,
Seki, M., Ohtsubo, E., and Maki, H. (1999) J. Mol. Biol. 289, 835850.
4. Breslauer, K. J., Frank, R., Blocker, H. and Marky, L. A. (1986) Proc. Natl. Acad. Sci. 83, 37463750.
5. Howley, P. M., Israel, M. A., Law, M. F. and Martin, M. A. (1979) J. Biol. Chem. 254, 48764883.
6. Cheng, S., Fockler, C., Barnes, W. M., and Higuchi, R. (1994) Proc. Natl. Acad. Sci. USA 91, 56955699.
7. Winship, P. R. (1989) Nucleic Acids Res. 17, 1266.
Appendix
Example amplifications
Amplification of 335 bp fragment (CFTR exon 11) from human genomic DNA
Reaction setup
Component
Volume
Final Concentration
10X Buffer for KOD Hot Start DNA
Polymerase
5 l
1X
25 mM MgSO4
3 l
1.5 mM
dNTPs (2 mM each)
5 l
0.2 mM (each)
PCR Grade Water
32 l
Sense (5') Primer (10 M)
1.5 l
0.3 M
Anti-Sense (3') Primer (10 M)
1.5 l
0.3 M
Human Genomic DNA* (100 ng/l)
1 l
2 ng/l
KOD Hot Start DNA Polymerase (1 U/l)
1 l
0.02 U/l
Total reaction volume
50 l
* Human Genomic DNA (Cat. No. 69237-3) diluted in TE to 100 ng/l
1
Cycling conditions
Step
Temperature and time
1. Polymerase activation
95C for 2 min
2. Denature
95C for 20 s
3. Annealing
56C for 10 s
4. Extension
70C for 4 s
Repeat steps 24
30 cycles
5. Hold
4C
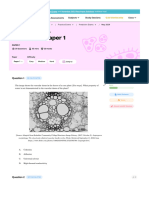
1.2 % TAE agarose gel
Lane 1 PCR Markers, 50 2000 bp
(Cat. No. 69278-3)
Lane 2 5 l PCR Reaction
USA and Canada
Tel (800) 628-8470
bioscienceshelp@
emdchemicals.com
Germany
Tel 0800 100 3496
techservice@merckbiosciences.de
United Kingdom and Ireland
UK Freephone 0800 622935
Ireland Toll Free 1800 409445
customer.service@merckbiosciences.co.uk
All Other Countries
www.merck4biosciences.com
bioscienceshelp@
emdchemicals.com
Cat. No.
Page
9 of 9
Page
User Protocol TB341 Rev. D 0111JN
Amplification of 919 bp ORF from plasmid DNA
Reaction setup
Component
Volume
Final Concentration
10X Buffer for KOD Hot Start DNA Polymerase
5 l
1X
25 mM MgSO4
3 l
1.5 mM
dNTPs (2 mM each)
5 l
0.2 mM (each)
PCR Grade Water
32 l
Sense (5') Primer (10 M)
1.5 l
0.3 M
Anti-Sense (3') Primer (10 M)
1.5 l
0.3 M
Plasmid DNA (10 ng/l, diluted in TE)
1 l
0.22 ng/l
KOD Hot Start DNA Polymerase (1 U/l)
1 l
0.02 U/l
Total reaction volume
50 l
Human Genomic DNA (Cat. No. 69237-3) diluted in TE to 100 ng/l
1
Cycling conditions
Step
Temperature and time
1. Polymerase activation
95C for 2 min
2. Denature
95C for 20 s
3. Annealing
55C for 10 s
4. Extension
70C for 15 s
Repeat steps 24
25 cycles
5. Hold
4C
1.4 % TAE agarose gel
USA and Canada
Tel (800) 628-8470
bioscienceshelp@
emdchemicals.com
Lane 1
PCR Markers, 502000 bp
(Cat. No. 69278-3)
Lane 2
5 l PCR Reaction
Germany
Tel 0800 100 3496
techservice@merckbiosciences.de
United Kingdom and Ireland
UK Freephone 0800 622935
Ireland Toll Free 1800 409445
customer.service@merckbiosciences.co.uk
All Other Countries
www.merck4biosciences.com
bioscienceshelp@
emdchemicals.com
You might also like
- Ott MannDocument1 pageOtt MannreveoNo ratings yet
- USF Fall 2015 PositionsDocument2 pagesUSF Fall 2015 PositionsreveoNo ratings yet
- Apple - Support - Confirmation 6:3:18Document2 pagesApple - Support - Confirmation 6:3:18reveoNo ratings yet
- RcrintroDocument184 pagesRcrintrorussell_mahmoodNo ratings yet
- Nature12149 s1Document21 pagesNature12149 s1reveoNo ratings yet
- RcrintroDocument184 pagesRcrintrorussell_mahmoodNo ratings yet
- Product No. Product Name Description Quantity Price Amount: Web - Ref (Webshop-Po-Number) : Www-53919Document1 pageProduct No. Product Name Description Quantity Price Amount: Web - Ref (Webshop-Po-Number) : Www-53919reveoNo ratings yet
- Nature 02872Document5 pagesNature 02872reveoNo ratings yet
- Chapter 1Document15 pagesChapter 1reveoNo ratings yet
- Nervous Conditions: A Critique of The Colonial Education SystemDocument6 pagesNervous Conditions: A Critique of The Colonial Education SystemreveoNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Sbstta 18 Inf 03 enDocument63 pagesSbstta 18 Inf 03 enPasta LoverNo ratings yet
- Cell Brochure by AlexDocument2 pagesCell Brochure by Alexapi-251002878No ratings yet
- Primer Design For PCR AssignmentDocument5 pagesPrimer Design For PCR Assignmentshalinianandhkumar100% (1)
- Fermentation Lab ReportDocument8 pagesFermentation Lab ReportSarah Sulon77% (13)
- Sector BiotechDocument109 pagesSector BiotechoptisearchNo ratings yet
- The - RNA World Hypothesis - A JournalDocument11 pagesThe - RNA World Hypothesis - A JournalAbhik Kumar HalderNo ratings yet
- GNIPST Bulletin 42.4Document20 pagesGNIPST Bulletin 42.4Gnipst BulletinNo ratings yet
- Central Dogma of Molecular BiologyDocument34 pagesCentral Dogma of Molecular BiologyGretz AnticamaraNo ratings yet
- Removing BT Eggplant From The Face of Indian Regulators: Nature Biotechnology September 2015Document5 pagesRemoving BT Eggplant From The Face of Indian Regulators: Nature Biotechnology September 2015weetengNo ratings yet
- Genetics, Lecture 11 (Lecture Notes)Document16 pagesGenetics, Lecture 11 (Lecture Notes)Ali Al-QudsiNo ratings yet
- The Physics of CancerDocument183 pagesThe Physics of CancerClaudio PerezNo ratings yet
- Viruses: Sars-Cov-2/Covid-19: Viral Genomics, Epidemiology, Vaccines, and Therapeutic InterventionsDocument18 pagesViruses: Sars-Cov-2/Covid-19: Viral Genomics, Epidemiology, Vaccines, and Therapeutic InterventionsMahantesh NyayakarNo ratings yet
- Genetics (Biol 105) Linkage and Recombination Exercises: Progeny Phenotype Progeny NumberDocument4 pagesGenetics (Biol 105) Linkage and Recombination Exercises: Progeny Phenotype Progeny NumberAngelica GeneraleNo ratings yet
- Introduction to Bioinformatics LabDocument3 pagesIntroduction to Bioinformatics LabRaj Kumar SoniNo ratings yet
- Qualiry Assurance Level 9Document31 pagesQualiry Assurance Level 9Abel YagoNo ratings yet
- Notes Carb MetabolismDocument7 pagesNotes Carb MetabolismCrimson MupasNo ratings yet
- Topic Wise List of Books For Plant Biotechnology and SciencesDocument8 pagesTopic Wise List of Books For Plant Biotechnology and SciencesAliNo ratings yet
- Biotechnology and Biological Preparations758578294Document24 pagesBiotechnology and Biological Preparations758578294venkats_001No ratings yet
- Student Exploration: RNA and Protein SynthesisDocument7 pagesStudent Exploration: RNA and Protein SynthesisMasonNo ratings yet
- Science 1987 Slamon 177 82 PDFDocument6 pagesScience 1987 Slamon 177 82 PDFAmin ArabNo ratings yet
- Microbes and Infection: Jin-Yan Li, Zhi You, Qiong Wang, Zhi-Jian Zhou, Ye Qiu, Rui Luo, Xing-Yi GeDocument6 pagesMicrobes and Infection: Jin-Yan Li, Zhi You, Qiong Wang, Zhi-Jian Zhou, Ye Qiu, Rui Luo, Xing-Yi GeLeah StroposNo ratings yet
- VAIBHAVDocument1 pageVAIBHAVUpadhayayAnkurNo ratings yet
- G8 Science Q4 Week 2 Cell Division Mitosis MeiosisDocument52 pagesG8 Science Q4 Week 2 Cell Division Mitosis MeiosisLeyannNo ratings yet
- Pharmaco VigilanceDocument31 pagesPharmaco VigilanceRasika NatuNo ratings yet
- Biochemistry - Module 1Document6 pagesBiochemistry - Module 1ricky fecaraNo ratings yet
- Bioenergetics Lesson 1Document37 pagesBioenergetics Lesson 1SalMaNaNo ratings yet
- ProlpDocument25 pagesProlpherryNo ratings yet
- IB Biology SL - 2024 Prediction Exam - May 2024 Paper 1Document16 pagesIB Biology SL - 2024 Prediction Exam - May 2024 Paper 1Christy HuynhNo ratings yet
- Q Topik 6Document32 pagesQ Topik 6Carin TanNo ratings yet
- Real Time PCRDocument10 pagesReal Time PCRHiciab Luismi CabreraNo ratings yet