Professional Documents
Culture Documents
2 Work Interactions
Uploaded by
mra3210Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
2 Work Interactions
Uploaded by
mra3210Copyright:
Available Formats
Work Interactions
IIT Bombay
ME 209
From mechanics,
Work = Force . Displacement
W F .ds
2
Introduction - Applications
Work Interactions (Tensile)
IIT Bombay
ME 209
Ultimate tensile strength
dW T .dL
Introduction - Applications
Work Interactions (Electrical)
IIT Bombay
ME 209
dW E.dq
Introduction - Applications
Work Interactions (Shaft)
IIT Bombay
ME 209
dW .d
http://www.youtube.com/watch?v=cEL7yc8R42k
Introduction - Applications
Work Interactions (Expansion)
IIT Bombay
ME 209
dW P.dV
http://www.youtube.com/watch?v=I-6AgvZOC-g&feature=related
Introduction - Applications
Work Interactions (contd.)
IIT Bombay
ME 209
system & surroundings
dW = X.dY
sign convention
Often, X = intensive property ;
Y = extensive property
One way / two way
Introduction - Applications
Thermodynamic definition of work
IIT Bombay
ME 209
Energy interaction between two systems
Reducible completely to raise of weight
Introduction - Applications
Thermodynamic definition of work (contd.)
IIT Bombay
ME 209
A1 A2
B1 B2
Is I work?
Is yes magnitude and direction?
Introduction - Applications
Thermodynamic definition of work (contd.)
IIT Bombay
ME 209
If possible, construct C1
A
A1 A2
C1
No change of state
h1
Introduction - Applications
Thermodynamic definition of work (contd.)
IIT Bombay
ME 209
If C1 can be constructed:
a. I is work
b. WA = +mgh1 ; WB = mgh1
c. A does work on B
Introduction - Applications
10
Thermodynamic definition of work (contd.)
IIT Bombay
ME 209
If construction of C1 is impossible, construct C2
C2
No change of state
h2
B
B1 B 2
Introduction - Applications
11
Thermodynamic definition of work (contd.)
IIT Bombay
ME 209
If C2 can be constructed:
a. I is work
b. WA = mgh2 ; WB = + mgh2
c. B does work on A
Introduction - Applications
12
Thermodynamic definition of work (contd.)
IIT Bombay
ME 209
If neither C1 or C2 can be constructed
I is not completely work
Introduction - Applications
13
Sign convention of Work
Work done BY system : positive
Work done ON system : negative
Introduction - Applications
IIT Bombay
ME 209
Mech. Engg.
Elec. Engg.
Physics
14
Quantification of work
IIT Bombay
ME 209
W Wexp Wshaft Welec Wother
A1 A2
2
W dWexp dWshaft dWelec Wother
2
W PdV d Edq Wother
Introduction - Applications
15
Quantification of work (contd.)
IIT Bombay
ME 209
dWany
1
can only be evaluated as an integral if the process
is Quasi-static
Introduction - Applications
16
Quantification of work (contd.)
P
IIT Bombay
ME 209
W is a path function
dW is not an exact differential
Hence dW is written as W, W, etc.
Introduction - Applications
17
Classification of Systems (based on Work)
IIT Bombay
ME 209
Systems can be classified by the number of two-way
interactions that can be executed:
Simple system (1)
Complex system (2)
Rudimentary system (0)
Introduction - Applications
18
Example 1
IIT Bombay
ME 209
An electrical heater has a resistance of 50. It is connected
across a power supply of 240V, for a period of 2 hours.
Determine the work done by the power supply on the heater.
How many units of electricity are consumed?
Introduction - Applications
19
Example 2
IIT Bombay
ME 209
A system containing 5 kg of a substance is stirred with a torque
of 0.3 kgf-m at a speed of 1000 rpm for 24 hours. The system
meanwhile expands from 1 m3 to 2 m3 against a constant
pressure of 4 kgf/cm2.
Determine the net work done in kJ.
Introduction - Applications
20
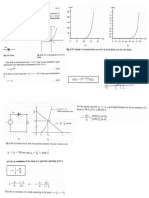
Example 3
IIT Bombay
ME 209
Consider the situation shown in the figure below. The width of the channel
is b, normal to the plane of the paper. The atmospheric pressure is P0.
Show that the force exerted by the water on the piston is
gh
F P0
hb
2
Calculate the work done by the water on the piston and by the
atmosphere on the water when the chamber length is slowly increased
from x1 to x2. Express your answer in terms of P0, b, h1, x1, x2, , and g.
Introduction - Applications
21
Example 4
IIT Bombay
ME 209
The pressure of a 250g block of metal is increased quasistatically and isothermally from 0 to 1000 bar. Assume the
density and isothermal bulk modulus of the metal remain
(almost) constant at 20 gm/cm2 and 2.1012 dyne/cm2
respectively.
Determine the work done in J. Isothermal bulk modulus is
defined as
P
V
Introduction - Applications
22
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- MainDocument4 pagesMainmra3210No ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Group 2, Pink, JayDocument3 pagesGroup 2, Pink, Jaymra3210No ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- AdahDocument5 pagesAdahmra3210No ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- HajhDocument4 pagesHajhmra3210No ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Group 2, Blue, JayDocument2 pagesGroup 2, Blue, Jaymra3210No ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- ReadmerDocument79 pagesReadmersatyajtiNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Usfu WeDocument1 pageUsfu Wemra3210No ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- MW 847Document2 pagesMW 847mra3210No ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Usfu WeDocument1 pageUsfu Wemra3210No ratings yet
- Internal Flows FolmulaeDocument1 pageInternal Flows Folmulaemra3210No ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- AddtwoDocument1 pageAddtwomra3210No ratings yet
- HJHUDQIDJDocument1 pageHJHUDQIDJmra3210No ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Ag AuerDocument1 pageAg Auermra3210No ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- AddtwoDocument1 pageAddtwomra3210No ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- Course Info BookletDocument34 pagesCourse Info BookletVipul sethNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Ee101 My Notes1Document2 pagesEe101 My Notes1mra3210No ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Git InstructionsDocument2 pagesGit Instructionsmra3210No ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- HJHUDQIDJDocument1 pageHJHUDQIDJmra3210No ratings yet
- Python TutorialDocument87 pagesPython TutorialCristiano PalmaNo ratings yet
- Firebase ServerDocument5 pagesFirebase Servermra3210No ratings yet
- Chapter 1Document22 pagesChapter 1nmanicbe76No ratings yet
- HJHUDQIDJDocument1 pageHJHUDQIDJmra3210No ratings yet
- Firebase ServerDocument5 pagesFirebase Servermra3210No ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Android NotesDocument2 pagesAndroid Notesmra3210No ratings yet
- Android NotesDocument2 pagesAndroid Notesmra3210No ratings yet
- Android NotesDocument2 pagesAndroid Notesmra3210No ratings yet
- Afgh 3Document7 pagesAfgh 3mra3210No ratings yet
- Afgh 1Document7 pagesAfgh 1mra3210No ratings yet
- Afgh 1Document7 pagesAfgh 1mra3210No ratings yet
- Thermo Dynamics Question BankDocument3 pagesThermo Dynamics Question Banknisar_ulNo ratings yet
- THE RIGHT APPROACH TO MF SELECTIONDocument2 pagesTHE RIGHT APPROACH TO MF SELECTIONMahesh NarayananNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- 03 Report Painter Report WriterDocument40 pages03 Report Painter Report Writerdeitron100% (3)
- SRB Session1 RofsDocument4 pagesSRB Session1 RofsFullo Flores MarviloneNo ratings yet
- Strength of Materials MarksDocument28 pagesStrength of Materials Markslogeshboy007No ratings yet
- Splunk Quick Reference GuideDocument6 pagesSplunk Quick Reference GuideLsniperNo ratings yet
- A Comparison of Subspace Methods For Sylvester Equations: Mathematics InstituteDocument9 pagesA Comparison of Subspace Methods For Sylvester Equations: Mathematics InstituteDurga SivakumarNo ratings yet
- Beales MethodDocument38 pagesBeales MethodAbani100% (3)
- World-GAN - A Generative Model For MinecraftDocument8 pagesWorld-GAN - A Generative Model For MinecraftGordonNo ratings yet
- Predicting Star Ratings Based On Annotated Reviews of Mobile AppsDocument8 pagesPredicting Star Ratings Based On Annotated Reviews of Mobile AppsAbdul KhaliqNo ratings yet
- Non Linear Static and Multi Axial Fatigue Analysis of Automotive Lower Control Arm Using NeinastranDocument11 pagesNon Linear Static and Multi Axial Fatigue Analysis of Automotive Lower Control Arm Using Neinastrangramesh1985No ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- ATOA CAE Multiphysics and Multimaterial Design With COMSOL Webinar PDocument31 pagesATOA CAE Multiphysics and Multimaterial Design With COMSOL Webinar PRaj C ThiagarajanNo ratings yet
- Exam Circular - HYE - C1 To 12, 2023-24Document26 pagesExam Circular - HYE - C1 To 12, 2023-24Aman ShethNo ratings yet
- Trigonometric Functions and Equations (Unit - 1)Document76 pagesTrigonometric Functions and Equations (Unit - 1)Santosh YaramatiNo ratings yet
- Decimal Differentiation by Monica LenewayDocument6 pagesDecimal Differentiation by Monica Lenewaygoldenoj100% (4)
- Rr10302 Applied MechanicsDocument12 pagesRr10302 Applied MechanicsSRINIVASA RAO GANTANo ratings yet
- 04 Handout 1 (Feedback)Document11 pages04 Handout 1 (Feedback)Kathleen Anne MendozaNo ratings yet
- UCC Computer Science student explores greedy algorithmsDocument3 pagesUCC Computer Science student explores greedy algorithmsEmmanuel EshunNo ratings yet
- 2.161 Signal Processing: Continuous and Discrete: Mit OpencoursewareDocument14 pages2.161 Signal Processing: Continuous and Discrete: Mit Opencoursewarelovelyosmile253No ratings yet
- 1e1: Engineering Mathematics I (5 Credits) LecturerDocument2 pages1e1: Engineering Mathematics I (5 Credits) LecturerlyonsvNo ratings yet
- BMS Sem 1 DSC Ge Sec Vac (Edit)Document27 pagesBMS Sem 1 DSC Ge Sec Vac (Edit)VISHESH 0009No ratings yet
- The Better Sine Wave IndicatorDocument10 pagesThe Better Sine Wave IndicatorcoachbiznesuNo ratings yet
- Operation ManagementDocument15 pagesOperation ManagementRabia RasheedNo ratings yet
- International Conference on Mathematical Advances and Applications Abstract BookDocument179 pagesInternational Conference on Mathematical Advances and Applications Abstract BookMUSTAFA BAYRAMNo ratings yet
- Transformation of Plane StressDocument27 pagesTransformation of Plane StressDave Harrison FloresNo ratings yet
- Bending Moment Normal Forces in Tunnel Linings PDFDocument8 pagesBending Moment Normal Forces in Tunnel Linings PDFhendrawanNo ratings yet
- Aircraft Radio SystemsDocument259 pagesAircraft Radio SystemsEdward Muriithi100% (2)
- Chapter Test 2 ReviewDocument23 pagesChapter Test 2 ReviewSheila Mae AsuelaNo ratings yet
- Unit 2. Error and The Treatment of Analytical Data: (1) Systematic Error or Determinate ErrorDocument6 pagesUnit 2. Error and The Treatment of Analytical Data: (1) Systematic Error or Determinate ErrorKamran JalilNo ratings yet
- Statistics___Probability_Q3__Answer_Sheet___1_.pdfDocument53 pagesStatistics___Probability_Q3__Answer_Sheet___1_.pdfJohn Michael BerteNo ratings yet