Professional Documents
Culture Documents
Amal Kebajikan1
Uploaded by
kjjkimkmk0 ratings0% found this document useful (0 votes)
10 views2 pageschem
Copyright
© © All Rights Reserved
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentchem
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
10 views2 pagesAmal Kebajikan1
Uploaded by
kjjkimkmkchem
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
You are on page 1of 2
|AMAL KEBAJIKAN S1/C1/01|kjj
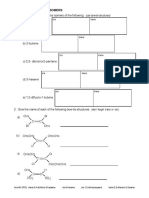
Isotope Notation
1. Uranium-235 and uranium-238 are considered isotopes of one another. How are uranium-
235 and uranium-238 similar, and how are they different?
2. Define isotope:
3. The number of protons in an atom is known as the ________________ of that atom.
4. The number of _______________ determines the name of the atom.
5. The nucleon number of an atom is the number of _____________ plus the number of
____________ in the nucleus of the atom.
6. The isotope notation for nitrogen-15 is as follows:
a. The number 15 is the _________ number.
b. The number 7 is the _________number.
c. How many neutrons does nitrogen-15 have? __________
7. Write the following in isotope notation:
a. zinc-66: _________ e. potassium-40: _________
b. chlorine-35: _________ f. silver-108: _________
c. plutonium-239: ________ g. thorium-234: _________
d. helium-4: _________ h. oxygen-16: _________
i. the atom with 12 protons and 12 neutrons ____________
j. the atom with 6 protons and 7 neutrons ____________
k. the atom with 79 protons and 117 neutrons. ____________
l. the phosphorus atom that has 17 neutrons. ____________
m. the copper atom that has 34 neutrons. ____________
on the iodine atom that has 72 neutrons ____________
8. Fill in the following table.
Nucleon Number
You might also like
- 4CH0 1C ChemistryDocument1 page4CH0 1C ChemistrykjjkimkmkNo ratings yet
- 4CH0 1C Rms ChemistryDocument32 pages4CH0 1C Rms ChemistryAlex Smith100% (1)
- Group Choice Activity - MoodleDocsDocument4 pagesGroup Choice Activity - MoodleDocskjjkimkmkNo ratings yet
- K2S2T3Document1 pageK2S2T3kjjkimkmkNo ratings yet
- Carbocation KeyDocument1 pageCarbocation KeykjjkimkmkNo ratings yet
- Set 3fDocument1 pageSet 3fkjjkimkmkNo ratings yet
- Kolej Matrikulasi KedahDocument2 pagesKolej Matrikulasi KedahkjjkimkmkNo ratings yet
- Kuliah K2S3Document4 pagesKuliah K2S3kjjkimkmkNo ratings yet
- Set 2Document2 pagesSet 2kjjkimkmkNo ratings yet
- Set 1Document1 pageSet 1kjjkimkmkNo ratings yet
- Isi KandunganDocument1 pageIsi KandungankjjkimkmkNo ratings yet
- K1S3T4ADocument1 pageK1S3T4AkjjkimkmkNo ratings yet
- Alkenes (Fasi)Document3 pagesAlkenes (Fasi)kjjkimkmkNo ratings yet
- Identifying Types of Reactions - KeyDocument3 pagesIdentifying Types of Reactions - KeykjjkimkmkNo ratings yet
- Chapter 16: Benzene - Electrophilic Aromatic Substitution: Chem231 Study Notes On McmurryDocument20 pagesChapter 16: Benzene - Electrophilic Aromatic Substitution: Chem231 Study Notes On McmurrykjjkimkmkNo ratings yet
- Al KanesDocument4 pagesAl KaneskjjkimkmkNo ratings yet
- Amalkebajikan Quiz Alkene OkDocument3 pagesAmalkebajikan Quiz Alkene OkkjjkimkmkNo ratings yet
- 24 - Organic Chemistry: Practice TestDocument2 pages24 - Organic Chemistry: Practice TestkjjkimkmkNo ratings yet
- Alkanes Who Am IDocument2 pagesAlkanes Who Am IkjjkimkmkNo ratings yet
- Carbo CationDocument1 pageCarbo CationkjjkimkmkNo ratings yet
- Ws 10.8 Geometric Isomers: (Use Bow-Tie Structures)Document1 pageWs 10.8 Geometric Isomers: (Use Bow-Tie Structures)kjjkimkmkNo ratings yet
- Structural Formulas and IUPAC Names of Phytane and Related AlkanesDocument2 pagesStructural Formulas and IUPAC Names of Phytane and Related AlkaneskjjkimkmkNo ratings yet
- Alkene Reactions: CH2N2 to AlcoholsDocument2 pagesAlkene Reactions: CH2N2 to AlcoholskjjkimkmkNo ratings yet
- Hydrocarbons ChapterDocument45 pagesHydrocarbons ChapterkjjkimkmkNo ratings yet
- Self-Study Worksheet III Isomerism ANSWERSDocument2 pagesSelf-Study Worksheet III Isomerism ANSWERSkjjkimkmkNo ratings yet
- 1hr AlkaneskmppDocument12 pages1hr AlkaneskmppkjjkimkmkNo ratings yet
- Studyon Alkane NameDocument3 pagesStudyon Alkane NamekjjkimkmkNo ratings yet
- Sk027 / Chapter 5: Hydrocarbon / Amalkebajikan01 / Nomenclature AlkaneDocument7 pagesSk027 / Chapter 5: Hydrocarbon / Amalkebajikan01 / Nomenclature AlkanekjjkimkmkNo ratings yet
- Alkane'S: - Alkanes Based On Molecular Weight - Isomeric Alkanes - Alkanes and CycloalkanesDocument1 pageAlkane'S: - Alkanes Based On Molecular Weight - Isomeric Alkanes - Alkanes and CycloalkaneskjjkimkmkNo ratings yet
- Physical & Chemical Properties of AlkanesDocument18 pagesPhysical & Chemical Properties of AlkaneskjjkimkmkNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)