Professional Documents
Culture Documents
QRQC Problem Solving Techniques
Uploaded by
Kristof MCCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
QRQC Problem Solving Techniques
Uploaded by
Kristof MCCopyright:
Available Formats
QRQC for non manufacturing processes
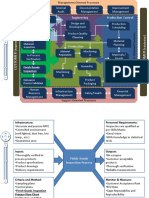
1 5W2H 2 GOOD AND BAD PART PROCESS VISUALIZATION 3 UNDERSTAND THE PROCESS BY VISUALIZATION
[3.8]
Describe the process by using Flow Charts, Process
[1.1]
Start Date: [1.2] QRQC Id:
GOOD PROCESS / GOOD PART ADDITIONAL INFORMATION
Maps, Sketches etc.
Part / Process
[1.3] Was the process performed according the
[3.1]
Name: standards (no rework or deviations from
procedure)?
[1.4]
Function [1.5]
Category
Where was the part produced / process
[3.2]
[1.6]QRQC-Leader:
QRQC-Team: performed?
5W2H
[1.7]
WHAT has happened? When was the part produced / process
[3.3]
performed?
[1.8]
WHY is it a problem? Do we detect the problem with our own
[3.4]
equipment?
[1.9]
WHEN was is detected? SINCE WHEN we have this problem? Which WABCO processes / people are involved
[3.5]
BAD PROCESS / BAD PART or might be affected?
[1.10]
WHERE was it detected? [3.6]
Did the problem occur before (recurrence)?
[1.11]
WHO detected it? In case of recurrence: What was the
[3.7]
countermeasure / corrective action?
[1.12]
HOW was it detected?
[1.13]
HOW many parts are affected?
[2.1]
What is the difference between a good and a bad part / process?
Team Leader: Name: Date:
[1.14]
Escalation Level:
(if necessary)
Regional/Functional Leader: Name: Date:
4 CONTAINMENT ACTIONS 5a
5 FACTOR TREE ANALYSIS (FTA): Why did the problem occur? 5b
7 FACTOR TREE ANALYSIS (FTA). Why didn't we detect the problem ?
[5.4]
Good part / [5.5]
Bad part / Good part / Bad part /
[4.1]
Containment Actions [4.2]
Who [4.3]
Plan date [4.4]
Status [5.1]
4M [5.2]
Factors [5.3]
Standard [5.6]
Difference 4M Factors Standard Difference
process process process process
No No
MATERIAL MATERIAL
Yes Yes
No No
Yes Yes
No No
METHOD METHOD
Yes Yes
No No
Yes Yes
No No
MAN MAN
Yes Yes
No No
Yes Yes
No No
MACHINE MACHINE
Yes Yes
No No
Yes Yes
No No
OTHERS OTHERS
Yes Yes
No No
Yes Yes
REPRODUCE THE FAILURE 5WHY ANALYSIS FOR OCCURENCE 5WHY ANALYSIS FOR NON-DETECTION
6a
7 6b
6a 6b
9
[6.1]
Reproduce the failure based on the significant factors found on FTA. If you could reproduce the failure, it
means that the Factor has a direct link with the problem.
[7.1]
Why did it happen? [7.3]
Why didn't we detect?
Describe what have you done to reproduce the failure: Why 1 : Why 1 :
Evidence 1 : Evidence 1 :
Why 2 : Why 2 :
Evidence 2 : Evidence 2 :
Results: Why 3 : Why 3 :
Evidence 3 : Evidence 3 :
Why 4 : Why 4 :
Evidence 4 : Evidence 4 :
In case you were not able to reproduce please escalate to next level and give reasons: Why 5 : Why 5 :
Evidence 5 : Evidence 5 :
[7.2]
Real Root Cause for [7.4]
Real Root Cause for
occurence non-detection
8 COUNTER MEASURES 9 Verification of Counter Measures and Sign Off
What is the amount of the deviation before and after the QRQC
[8.1]
Permanent Corrective Actions [8.2]
Who [8.3]
Plan date [8.4]
Status
[9.1]
Insert Graph Titel
Insert Graph Titel Here
11
10
9
Quantity
0 Date
[8.5]
Update documents:
Actions
Documented Procedure Date + 1 Date + 2 Date + 3 Date + 4 Date + 5 Date + 6 Date + 7
Implemented
Work Instructions [9.2]
Date
P/D-FMEA [9.3]
Quantity
TControl Plan *See instructions for detailed information
H JED
[9.4]
Comments:
ILessons Learned Card
N
TOthers (specify):
K
H [9.5] QRQC closed!
QRQC closed!
[8.6]
Process Owner
I Sign-off:
Name: Sign-off: QRQC Leader
Name: Sign-off: Team Leader Name: Sign-off: Date:
u
N
Sign-off:
Sign-off:
n
K
t
iu
ln
lt
You might also like
- Reverse PFMEA Worksheet 2Document1 pageReverse PFMEA Worksheet 2adochan91% (11)
- QRQC / 8D Form: D 1. Description of The ProblemDocument5 pagesQRQC / 8D Form: D 1. Description of The ProblemDearRed FrankNo ratings yet
- Control Plan Audit FormDocument2 pagesControl Plan Audit Formdpcastilla50% (2)
- SAFE LAUNCH ProcedureDocument2 pagesSAFE LAUNCH ProcedurePRAMOD86% (7)
- Training Module 8D QRCIDocument81 pagesTraining Module 8D QRCIMAPROLProjetos100% (6)
- IATF 16949 Documentation Toolkit GuideDocument4 pagesIATF 16949 Documentation Toolkit GuideOscar Solis MartirNo ratings yet
- QRQC - Quick Response To Quality ControlDocument49 pagesQRQC - Quick Response To Quality ControlKshitij Bhakoo100% (4)
- AME Lean AssessmentDocument35 pagesAME Lean AssessmentNainerd Jing Jing100% (1)
- Human Error Root Cause Analisys - HercaDocument6 pagesHuman Error Root Cause Analisys - HercaSamuel Colaço100% (3)
- 8D QRQC problemDocument1 page8D QRQC problemJordi Palomar100% (5)
- Uk Vec Pdca QRQC PrésentationDocument149 pagesUk Vec Pdca QRQC Présentationmarsan1708100% (1)
- Process Capability Study TemplateDocument3 pagesProcess Capability Study Templateapi-3852736100% (4)
- GM 1927-33 GM Global GP-12 Audit March 13, 2008Document8 pagesGM 1927-33 GM Global GP-12 Audit March 13, 2008Neumar NeumannNo ratings yet
- Reverse PFMEADocument5 pagesReverse PFMEASantosh BallalNo ratings yet
- Processaudit VDA 6.3 - RecentDocument34 pagesProcessaudit VDA 6.3 - Recentavinash_k007No ratings yet
- QRQCDocument1 pageQRQCVirginia GrandoNo ratings yet
- QRQC Training ProtocolDocument52 pagesQRQC Training ProtocolSudhagar P100% (1)
- UK VEC PDCA QRQC PrésentationDocument149 pagesUK VEC PDCA QRQC Présentationlaurasirbu100% (3)
- Smed FormsDocument11 pagesSmed Formsokr15100% (1)
- The 7QB of Production: FAS Training PresentationDocument50 pagesThe 7QB of Production: FAS Training Presentationcong da100% (1)
- DMAIC Tool SummaryDocument1 pageDMAIC Tool SummaryGautam GoyalNo ratings yet
- Lean at AlstomDocument22 pagesLean at AlstomSrini Vsan100% (1)
- System QSB First Step Fast Response PDFDocument19 pagesSystem QSB First Step Fast Response PDFPrabagarane RamachandranNo ratings yet
- Measure a factory's ability to meet production targets with OPRDocument10 pagesMeasure a factory's ability to meet production targets with OPRalbertoNo ratings yet
- E-Book - 8D Problem SolvingDocument33 pagesE-Book - 8D Problem SolvingAd Nobre100% (1)
- Cqi 8 Layered Process Audits GuidelineDocument2 pagesCqi 8 Layered Process Audits GuidelinePraveen Malavae33% (3)
- Leadership Today Practices For Personal and Professional PerformanceDocument412 pagesLeadership Today Practices For Personal and Professional PerformanceEstie100% (4)
- Good Process / Good Part: QRQC For Non Manufacturing ProcessesDocument1 pageGood Process / Good Part: QRQC For Non Manufacturing ProcessesVeung SingkhekNo ratings yet
- The goal is to protect the customer in the short term while the root cause is investigated. Actions should address existing non-conforming productDocument50 pagesThe goal is to protect the customer in the short term while the root cause is investigated. Actions should address existing non-conforming productPamfeel1100% (1)
- QRQC Training ModuleDocument37 pagesQRQC Training Modulemoez100% (1)
- Supplier QRQC ImplementationDocument21 pagesSupplier QRQC ImplementationChristopher GILL100% (1)
- 8 QRQC Working MethodDocument14 pages8 QRQC Working MethodLuis Cisneros100% (1)
- Standardized Work Combination FormDocument3 pagesStandardized Work Combination FormisolongNo ratings yet
- GM 1927-30 QSB AuditDocument38 pagesGM 1927-30 QSB Auditmanune01No ratings yet
- Life System Qrqc-Monitoring Coaching v0 3Document5 pagesLife System Qrqc-Monitoring Coaching v0 3api-334903105No ratings yet
- QRQC PPTDocument10 pagesQRQC PPTNirmalya MishraNo ratings yet
- Core Tools (APQP, PPAP, FMEA, MSA, SPC and Problem Solving)Document2 pagesCore Tools (APQP, PPAP, FMEA, MSA, SPC and Problem Solving)skluxNo ratings yet
- Reverse FMEA Training Ensures Continuous Process ImprovementDocument2 pagesReverse FMEA Training Ensures Continuous Process ImprovementIonut Eduard100% (2)
- Lpa FormDocument2 pagesLpa FormEngineer100% (2)
- Daimler-Chrysler Layered Process Audits (DCX Lpa) : Alberta RisnerDocument23 pagesDaimler-Chrysler Layered Process Audits (DCX Lpa) : Alberta Risneralberto100% (1)
- Manual Alumno QRQC v2Document35 pagesManual Alumno QRQC v2Carlos AguilarNo ratings yet
- Presentation of The NSA: New Supplier AssessmentDocument35 pagesPresentation of The NSA: New Supplier AssessmentMojtaba MousaviNo ratings yet
- 5S Routine Audit Form: Quit Created by Javier RubioDocument7 pages5S Routine Audit Form: Quit Created by Javier RubioLa MetalurgicaNo ratings yet
- QRQC Manual: Quick Response Quality ControlDocument36 pagesQRQC Manual: Quick Response Quality ControlCarlos Aguilar100% (1)
- TPM 20guideline 20ver2Document20 pagesTPM 20guideline 20ver2Pedro SilvaNo ratings yet
- Standard Work For Leaders - YescoDocument43 pagesStandard Work For Leaders - YescoPavel Collado100% (3)
- Top Lean Tools - Top 26 Lean Manufacturing Tools - ExamplesDocument18 pagesTop Lean Tools - Top 26 Lean Manufacturing Tools - ExamplesrohitjandialNo ratings yet
- Quick Response Quality Control QRQCDocument17 pagesQuick Response Quality Control QRQCKristof MC100% (1)
- 02 - Fast Response - QIP V3Document85 pages02 - Fast Response - QIP V3HOSSIENNo ratings yet
- Formel Q Quality Capability Supplier Assessment Guidelines 2005 PDFDocument152 pagesFormel Q Quality Capability Supplier Assessment Guidelines 2005 PDFAntonioNo ratings yet
- Poka-Yoke Lean StrategyDocument82 pagesPoka-Yoke Lean Strategypablo7890100% (1)
- PCM SLP - Introduction: PreparationDocument12 pagesPCM SLP - Introduction: PreparationDavid MorenoNo ratings yet
- Daimler Process Signoff DocumentDocument228 pagesDaimler Process Signoff DocumentWolf Villarin100% (1)
- Operations: Group Leader: Daily Department Manager: Weekly Plant Manager: MonthlyDocument4 pagesOperations: Group Leader: Daily Department Manager: Weekly Plant Manager: MonthlyR JNo ratings yet
- LPA - Layer Process Audit GuidelinesDocument23 pagesLPA - Layer Process Audit GuidelinesR J100% (2)
- Reverse Fmea: AMDEC InverséeDocument12 pagesReverse Fmea: AMDEC InverséeMoez Aloui100% (2)
- Error Proofing TechniquesDocument140 pagesError Proofing TechniquesThe Informative Corner हिंदीNo ratings yet
- Core Tools Forms V5Document128 pagesCore Tools Forms V5hudelvillar100% (1)
- Lean Six Sigma Leadership 1615402359Document1 pageLean Six Sigma Leadership 1615402359Kristof MCNo ratings yet
- 5Ss 1615573743Document48 pages5Ss 1615573743Kristof MCNo ratings yet
- LSS Templates - M Cox - v4 Aug 08Document139 pagesLSS Templates - M Cox - v4 Aug 08Kristof MCNo ratings yet
- Business Ethics PDFDocument37 pagesBusiness Ethics PDFtubuiNo ratings yet
- 5S Handbook PDFDocument20 pages5S Handbook PDFAnonymous iMq2HDvVqNo ratings yet
- External Auditing and QualityDocument351 pagesExternal Auditing and QualityKristof MC100% (1)
- 5 Ss Visual ControlDocument57 pages5 Ss Visual ControlR.BALASUBRAMANINo ratings yet
- 5Ss 1615573784Document20 pages5Ss 1615573784Kristof MCNo ratings yet
- 5S Guide: An Introduction To The 5S Method and Practical Tips For Implementation in Any FacilityDocument25 pages5S Guide: An Introduction To The 5S Method and Practical Tips For Implementation in Any FacilityAndré Luiz LimaNo ratings yet
- Yield GE Wekk48 - 52Document10 pagesYield GE Wekk48 - 52Kristof MCNo ratings yet
- Dmaic Roadmap PDFDocument1 pageDmaic Roadmap PDFKristof MCNo ratings yet
- Black Belt Tollgate Checklist BBv1.1Document12 pagesBlack Belt Tollgate Checklist BBv1.1Kristof MCNo ratings yet
- Development and Implementatio A Remote Audit Tool For HighDocument92 pagesDevelopment and Implementatio A Remote Audit Tool For HighKristof MCNo ratings yet
- TPS - The Four Elements of Built in QualityDocument5 pagesTPS - The Four Elements of Built in QualityKristof MCNo ratings yet
- Effective Root Cause Analysis with 3 Legged 5 WhyDocument42 pagesEffective Root Cause Analysis with 3 Legged 5 WhySerchecko JaureguiNo ratings yet
- LSSBBDocument35 pagesLSSBBKristof MCNo ratings yet
- Potential Causes of Quality Issues in Composite ManufacturingDocument23 pagesPotential Causes of Quality Issues in Composite ManufacturingKristof MCNo ratings yet
- Policy Deployment X-MatrixDocument12 pagesPolicy Deployment X-MatrixKristof MC100% (1)
- Plantilla Modelo Hoshin KanriDocument8 pagesPlantilla Modelo Hoshin KanriJavier NumpaqueNo ratings yet
- Akashi Demand LetterDocument1 pageAkashi Demand LetterKristof MCNo ratings yet
- Policy Deployment X-MatrixDocument12 pagesPolicy Deployment X-MatrixKristof MC100% (1)
- Special Characteristics PDFDocument11 pagesSpecial Characteristics PDFKristof MCNo ratings yet
- LSSBBDocument35 pagesLSSBBKristof MCNo ratings yet
- Rakusa Simona PDFDocument147 pagesRakusa Simona PDFjames007spyNo ratings yet
- ISO Checklist of Mandatory Documentation Required by IATF 16949 en YES OKDocument20 pagesISO Checklist of Mandatory Documentation Required by IATF 16949 en YES OKroelly100% (2)
- Learn To SeeDocument67 pagesLearn To SeeKristof MCNo ratings yet
- Feleffektsanalys/Failure Mode and Effect Analysis Konstruktion/DesignDocument12 pagesFeleffektsanalys/Failure Mode and Effect Analysis Konstruktion/DesignKristof MCNo ratings yet
- Attribute Gage RR RidgwayDocument50 pagesAttribute Gage RR RidgwayPradeepNo ratings yet
- Checklist of Mandatory Documentation Required by IATF 16949 enDocument3 pagesChecklist of Mandatory Documentation Required by IATF 16949 enKristof MC100% (1)
- Function X - A Universal Decentralized InternetDocument24 pagesFunction X - A Universal Decentralized InternetrahmahNo ratings yet
- Dir x1560 Datasheet Eu enDocument2 pagesDir x1560 Datasheet Eu enMilosNo ratings yet
- 15 Reasons To Use Redis As An Application Cache: Itamar HaberDocument9 pages15 Reasons To Use Redis As An Application Cache: Itamar Haberdyy dygysd dsygyNo ratings yet
- Eclipse PDFDocument18 pagesEclipse PDFanjaniNo ratings yet
- Simple Way To Load Small Chunks of Data As You Scroll in Angular - by Ramya Balasubramanian - Geek Culture - Oct, 2021 - MediumDocument9 pagesSimple Way To Load Small Chunks of Data As You Scroll in Angular - by Ramya Balasubramanian - Geek Culture - Oct, 2021 - MediumRanjana PatilNo ratings yet
- Saas Solutions On Aws FinalDocument26 pagesSaas Solutions On Aws Finalfawares1No ratings yet
- Scripting Language - WikipediaDocument9 pagesScripting Language - WikipediaGilbertNo ratings yet
- A Technical Seminar ReportDocument28 pagesA Technical Seminar Reportnishitha pachimatlaNo ratings yet
- GUIDDocument215 pagesGUIDPrakashNo ratings yet
- KPT ShapeShifterDocument28 pagesKPT ShapeShifterLuis TovarNo ratings yet
- Register & Install Synthogy ProductsDocument8 pagesRegister & Install Synthogy ProductsEduardo MontielNo ratings yet
- Fertilizer Information System For Banana PlantatioDocument5 pagesFertilizer Information System For Banana PlantatioHazem EmadNo ratings yet
- One Pager v2Document2 pagesOne Pager v2JuanCarlosMarrufoNo ratings yet
- CGI in Movies vs Video GamesDocument21 pagesCGI in Movies vs Video GamesAbdul MajidNo ratings yet
- Pervasive Healthcare Computing: EMR/EHR, Wireless and Health MonitoringDocument3 pagesPervasive Healthcare Computing: EMR/EHR, Wireless and Health MonitoringPajak RedikonNo ratings yet
- FLS DemonstrationDocument30 pagesFLS DemonstrationLeah UljerNo ratings yet
- Conversor para PFO (Fibra Optica Plastica)Document2 pagesConversor para PFO (Fibra Optica Plastica)madmax258No ratings yet
- Test Script 459680Document9 pagesTest Script 459680Naresh KumarNo ratings yet
- Schedule SPC Training ProcessDocument10 pagesSchedule SPC Training ProcessTin NguyenNo ratings yet
- SPDIF WhitepaperDocument14 pagesSPDIF Whitepaperapi-3760834100% (1)
- Midterm I Review - FinalDocument25 pagesMidterm I Review - FinalFarah TarekNo ratings yet
- Addons Mozilla Org en US Firefox Addon Save As PDF Utm SourcDocument5 pagesAddons Mozilla Org en US Firefox Addon Save As PDF Utm SourcThanga PandiNo ratings yet
- MATM111 (Revised2)Document12 pagesMATM111 (Revised2)Zyra PascualNo ratings yet
- Kyocera TASKalfa 3501i BrochureDocument8 pagesKyocera TASKalfa 3501i Brochuretaunweer2No ratings yet
- Final Year Project TopicsDocument112 pagesFinal Year Project TopicsJyothi BurlaNo ratings yet
- Hive Tutorial PDFDocument14 pagesHive Tutorial PDFbewithyou2003No ratings yet
- Tutorial - Change The OS X Version by Modifying Systemversion - PlistDocument17 pagesTutorial - Change The OS X Version by Modifying Systemversion - PlistIsrael Jimenez AmorizNo ratings yet
- Stock Coal and Limestone Feed Systems: Powering Industry ForwardDocument12 pagesStock Coal and Limestone Feed Systems: Powering Industry ForwardAchmad Nidzar AlifNo ratings yet
- Chapter 6-Sensors-Different Types of SensorsDocument23 pagesChapter 6-Sensors-Different Types of SensorsbettyNo ratings yet