Professional Documents
Culture Documents
Clinical trial peer review and drug information resources
Uploaded by
agent2cat0 ratings0% found this document useful (0 votes)
107 views2 pagesThis document provides information on drug information resources and medication errors. It outlines primary, secondary, and tertiary sources for drug information including scientific journals, indexes, textbooks, and compendiums. Various compendiums and databases are also described that contain drug monographs and clinical information. Types of medication errors such as prescribing errors, dosing errors, and administration errors are defined. Guidelines for taking certain drugs with food, water, or an empty stomach are also summarized.
Original Description:
Drug information sources - PEBC EE preparation
Original Title
58 Drug Information Resources
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document provides information on drug information resources and medication errors. It outlines primary, secondary, and tertiary sources for drug information including scientific journals, indexes, textbooks, and compendiums. Various compendiums and databases are also described that contain drug monographs and clinical information. Types of medication errors such as prescribing errors, dosing errors, and administration errors are defined. Guidelines for taking certain drugs with food, water, or an empty stomach are also summarized.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
107 views2 pagesClinical trial peer review and drug information resources
Uploaded by
agent2catThis document provides information on drug information resources and medication errors. It outlines primary, secondary, and tertiary sources for drug information including scientific journals, indexes, textbooks, and compendiums. Various compendiums and databases are also described that contain drug monographs and clinical information. Types of medication errors such as prescribing errors, dosing errors, and administration errors are defined. Guidelines for taking certain drugs with food, water, or an empty stomach are also summarized.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
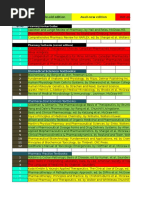
DRUG INFORMATION RESOURCES
Peer review: assessment of clinical trial by experts for scientific merit, participant safety, ethics
SOURCES
Primary Original information about clinical Scientific journals (Pharmacy
trials/research Connection, Canadian Family
Most current information keeps up Physician, Canadian Pharmacy Journal)
with latest developments & research
in pharmacy
Source of continuing education
Does not guarantee article accuracy,
but good info credibility
Secondary Info from primary sources compiled Indexes (Clin-Alert, Index Medicus),
as abstracts and published in bibliography, abstracts, internet
secondary sources search, Medline/PubMed
Quick, selective screening of primary
literature for specific info
Each indexing service may provide
specific list of journals, which can limit
thoroughness of literature search
Tertiary Obtained from primary/secondary Textbooks, compendia (CPS, US
sources pharmacopoeia, Martindale, Mother
Easy, comprehensive topics in 1 book risk, Remington, RxTx, Cochrane
No recent information database, Health Canada Drug Product
Database)
Compendium of Drug monographs, s/e, clinical uses, interactions, contraindications,
Pharmaceutical Specialty pharmacology, dosing, storage info, drugs containing allergens
(CPS or RxTx) (peanut, soy, ethanol), immunization schedule
Compendium of Treatment options (1st line, alternatives)
Therapeutic Choices (CTC)
Compendium of Self care, OTC
Therapeutic Minor
Ailments (CTMA)
US approved FDA drugs
USP-DI Vol 1 Vol 3: Orange book: Approved drug products with therapeutic
equivalence evaluations
Cochrane database Evidence-based medicine systematic review
Health Canada DPD Newly approved drugs
Medline/Pubmed Off-label drugs
Martindale Drugs manufactured in foreign countries, off-label drugs
Handbook of Injectable IV mixture compatibility
Drugs
Remington: Science and Pharmaceutics, therapeutics, pharmacy practice, industrial
Practice of Pharmacy Compounding, buffers, excipients
Compounding: Remingtons, Merck index Pregnancy, Lactation: Mother Risk
MEDICATION ERRORS
Health Canada MedEffect Drug advisories, withdrawals, recalls
Health Canada Pharmacovigilance ADR in post-marketing surveillance
Canadian Institute of Health Information (CIHI) Medication incidences in hospital
Institute of Safe Medication Practices (ISMP) Medication incidences in community
Canadian Patient Safety Institute (CPSI) Promotes innovative solutions & facilitates
collaboration among government &
stakeholders to enhance patient safety
Drug recalls
Class/Type I Class/Type II Class/Type III
Strong likelihood that product May cause temporary but Product not likely to cause
will cause serious ADR/death reversible adverse; small ADR
chance of ADR
Types of medication errors
Prescribing Wrong dosage form Deterioration
Omission Wrong Monitoring
Wrong time administration Compliance
Unauthorized drug Wrong drug Other
Improper dose preparation
Take w/ plenty of water:
Sulphonamides ( chance of crystalluria)
Expectorants
Bulk laxatives
Irritating drugs (theophylline, K supplements, some antibiotics)
Take w/food or milk
NSAIDs, ASA
Erythromycin
Nitrofurantoin
Valproic acid
Take on empty stomach (1h before or 2-3h after food)
Levothyroxine
Ampicillin
Tetracycline
You might also like
- Ed enDocument376 pagesEd enNashria Rusdhy100% (2)
- Medical Slang, Acronyms and Veterinary TermsDocument26 pagesMedical Slang, Acronyms and Veterinary TermsAneez Abdulsamad100% (1)
- Last 173 Q 8/2017Document25 pagesLast 173 Q 8/2017ahmed masoud100% (1)
- All Other ClassificationsDocument6 pagesAll Other ClassificationsCorey100% (1)
- Drugs To Watch With WARFARINDocument3 pagesDrugs To Watch With WARFARINRajendra RaiNo ratings yet
- Formulas Eval 2018Document5 pagesFormulas Eval 2018bhaveshNo ratings yet
- Naplex Mpje: 2017 Candidate Registration BulletinDocument51 pagesNaplex Mpje: 2017 Candidate Registration BulletinLam LamNo ratings yet
- 2017 April Exam CompilationDocument8 pages2017 April Exam CompilationabbasyaqobiNo ratings yet
- FPGEC Application Guide SummaryDocument6 pagesFPGEC Application Guide SummaryStephanie Camille Samonte100% (1)
- Refer Books Sort ListDocument12 pagesRefer Books Sort ListDr-Ram ChowdharyNo ratings yet
- Canadian Pharmacy Review Ver1Document6 pagesCanadian Pharmacy Review Ver1Mon MonNo ratings yet
- Mock TestDocument20 pagesMock Testironcahir61100% (1)
- Hester PediatricEthicsFinalProofsDocument9 pagesHester PediatricEthicsFinalProofsFitria FitriaNo ratings yet
- PEBC EE BlueprintDocument2 pagesPEBC EE BlueprintcoolchapNo ratings yet
- LAW Drug Schedules and Prescribing Authority GuideDocument19 pagesLAW Drug Schedules and Prescribing Authority GuideabbasyaqobiNo ratings yet
- The Loss of Sadness (2007) HorwitzDocument304 pagesThe Loss of Sadness (2007) HorwitzSpongeBobLongPants100% (3)
- Naplex Mpje Bulletin May 14 2018Document50 pagesNaplex Mpje Bulletin May 14 2018Clayton JensenNo ratings yet
- The Use of Restraints and Psychotropic Medications in People With DementiaDocument36 pagesThe Use of Restraints and Psychotropic Medications in People With DementiaSBS_NewsNo ratings yet
- 4 Competency 4 Mock Test May 2013Document38 pages4 Competency 4 Mock Test May 2013Andrew MeNo ratings yet
- Matching ActivityDocument26 pagesMatching Activityapi-661456802No ratings yet
- Pebc CompilationDocument14 pagesPebc CompilationAarti AroraNo ratings yet
- OTC Exam 2 Study GuideDocument32 pagesOTC Exam 2 Study GuideDave WinNo ratings yet
- Applying For Document Evaluation - PEBCDocument17 pagesApplying For Document Evaluation - PEBCSiraj AliNo ratings yet
- CLEAR 2005 - Scores and Reports3 - CObyrneLilaDocument21 pagesCLEAR 2005 - Scores and Reports3 - CObyrneLilaMithNo ratings yet
- 1 QE Therapeutic Mock Test 4 QA Nov 201371Document68 pages1 QE Therapeutic Mock Test 4 QA Nov 201371Andrew MeNo ratings yet
- Apc0271 V4 PDFDocument53 pagesApc0271 V4 PDFDrAnisha PatelNo ratings yet
- RC msn2Document204 pagesRC msn2Amit Pasi100% (1)
- Pharmacy: Undergraduate Study 2016Document18 pagesPharmacy: Undergraduate Study 2016kgiyerNo ratings yet
- Ebersole and Hess Gerontological Nursing and Healthy Aging 5th Edition Touhy Test BankDocument8 pagesEbersole and Hess Gerontological Nursing and Healthy Aging 5th Edition Touhy Test Bankpatriciaoneillozmpwqbdra100% (16)
- Immunization GuideDocument26 pagesImmunization GuideRob CrosbyNo ratings yet
- The Pharmacists' Guide to Selling Their Business: An Essential Exit Planning Resource for Canadian Independent Pharmacy OwnersFrom EverandThe Pharmacists' Guide to Selling Their Business: An Essential Exit Planning Resource for Canadian Independent Pharmacy OwnersNo ratings yet
- WHO (World Health Organization)Document6 pagesWHO (World Health Organization)Hilmi MusaNo ratings yet
- Unit 5 Bioethics BSNDocument25 pagesUnit 5 Bioethics BSNTamara Kate HalicanNo ratings yet
- Ctma 2018Document1,161 pagesCtma 2018Eldhose KuriakoseNo ratings yet
- Naplex Mpje BulletinDocument49 pagesNaplex Mpje BulletinAssignment Abroad0% (1)
- Antidotes and The Clinical Applications Antidote: An Antidote Is A Substance Which Can Counteract A Form ofDocument2 pagesAntidotes and The Clinical Applications Antidote: An Antidote Is A Substance Which Can Counteract A Form ofManohar Chowdary KovvuriNo ratings yet
- Epp5 Fall 2020 The Practice of Pharmacy in Florida - Laws Rules - Alvarez Student Version 3 SlidesDocument25 pagesEpp5 Fall 2020 The Practice of Pharmacy in Florida - Laws Rules - Alvarez Student Version 3 Slidesapi-552486649No ratings yet
- OSCE - Sample Chapter PDFDocument32 pagesOSCE - Sample Chapter PDFAndrés LLanos PrietoNo ratings yet
- APHA-Chapter-34 - Patient Assessment Laboratory: REVIEW OF SYSTEMS - Physical Assessment, Vital Signs& ObservationsDocument13 pagesAPHA-Chapter-34 - Patient Assessment Laboratory: REVIEW OF SYSTEMS - Physical Assessment, Vital Signs& ObservationsDrSamia El WakilNo ratings yet
- 2015 2016 College of Pharmacy Student HandbookDocument62 pages2015 2016 College of Pharmacy Student HandbookFiya AwanNo ratings yet
- Careers in PharmacyDocument6 pagesCareers in PharmacyChaitanya GaddeNo ratings yet
- Guidelines Minimum Standard Pharmacies HospitalsDocument11 pagesGuidelines Minimum Standard Pharmacies HospitalsEngyKamalNo ratings yet
- Gateway:: The Fee For Enrolment in The Gateway Is $340Document5 pagesGateway:: The Fee For Enrolment in The Gateway Is $340Farhan aliNo ratings yet
- Studying RoutineDocument12 pagesStudying RoutineKelvin Lim Wei Liang100% (1)
- Pharmacist 3Document38 pagesPharmacist 3AHAMED SHIFAANNo ratings yet
- Pharmacist Role in Dispensing Medicine PDFDocument3 pagesPharmacist Role in Dispensing Medicine PDFNavi JcNo ratings yet
- Top 100 Drugs in CanadaDocument25 pagesTop 100 Drugs in CanadaMohamed Omer100% (1)
- Bonner CHPT 04-339605c0rdzDocument10 pagesBonner CHPT 04-339605c0rdzSimon LaiNo ratings yet
- Confuseddrugnames 201902Document11 pagesConfuseddrugnames 201902Detya PertiwiNo ratings yet
- CoursesDocument2 pagesCoursesapi-586042393No ratings yet
- Feb 2018 ExamDocument10 pagesFeb 2018 Examreethu mammenNo ratings yet
- Oman Prometric Exam Notes: Download NowDocument16 pagesOman Prometric Exam Notes: Download NowneethuNo ratings yet
- Cal Pharmaspirit PH Cal Q ADocument49 pagesCal Pharmaspirit PH Cal Q AAriadne BalmacedaNo ratings yet
- Foreign Grads Info LetterDocument3 pagesForeign Grads Info LetterRoman WinerNo ratings yet
- HAAD Applicant KitDocument9 pagesHAAD Applicant KitMenGuitarNo ratings yet
- Pebc StatisticsDocument1 pagePebc Statisticscesar138No ratings yet
- KAPS Pharmacist Syllabus The PharmapediaDocument3 pagesKAPS Pharmacist Syllabus The PharmapediaSanam ThahaNo ratings yet
- PEBCHandbookDocument15 pagesPEBCHandbookversion1403No ratings yet
- Part A: Job Analysis Information Sheet of Hospital PharmacistDocument3 pagesPart A: Job Analysis Information Sheet of Hospital PharmacistTan Su SuanNo ratings yet
- Pharmacist Exam References & ResourcesDocument4 pagesPharmacist Exam References & Resourcesmahmoud jalloulNo ratings yet
- Prometric BooksDocument1 pagePrometric Bookssumaiya jalal100% (1)
- Insulin Chart: Insulin Type Onset of Action Peak Duration of ActionDocument1 pageInsulin Chart: Insulin Type Onset of Action Peak Duration of ActionGeorge ZachariahNo ratings yet
- CDM Sample Resume 3Document4 pagesCDM Sample Resume 3Rahul SNo ratings yet
- HOSPITAL AND CLINICAL PHARMCAY QuestionsDocument20 pagesHOSPITAL AND CLINICAL PHARMCAY Questionslola&losa farhanNo ratings yet
- M. Pharm Review NAPLEX07Document1 pageM. Pharm Review NAPLEX07JUSASBNo ratings yet
- Transcription factors and gene regulationDocument39 pagesTranscription factors and gene regulationRicky100% (1)
- Quotes On VaccinesDocument6 pagesQuotes On VaccinesKris BargerNo ratings yet
- 50 Years AESGPDocument28 pages50 Years AESGPaesgpNo ratings yet
- IEHP Authorization H2309444702 UM Tran Auth Form ServicingDocument3 pagesIEHP Authorization H2309444702 UM Tran Auth Form ServicingRo DanielaNo ratings yet
- Quality DepartmentDocument1 pageQuality DepartmentgsaldadzeNo ratings yet
- Status of Compliance With Generics ActDocument4 pagesStatus of Compliance With Generics ActKevin Nave RiveraNo ratings yet
- Wvsu MC Org ChartDocument1 pageWvsu MC Org ChartquesterNo ratings yet
- Office Memorandum Regarding Linking of CGHS Beneficiary ID With The ABHA (Jan 2023)Document4 pagesOffice Memorandum Regarding Linking of CGHS Beneficiary ID With The ABHA (Jan 2023)Aakash SinghNo ratings yet
- Research Article 1Document13 pagesResearch Article 1Nicole BamentNo ratings yet
- Endodontic Treatment FailureDocument8 pagesEndodontic Treatment FailureHawzheen SaeedNo ratings yet
- Apremilast PI Europe Aug 2019Document14 pagesApremilast PI Europe Aug 2019Wei Sheng ChongNo ratings yet
- ANC SreelakshmiDocument22 pagesANC SreelakshmiEmyNo ratings yet
- 3D CD Technique Completes Dentures in 3 Days for ElderlyDocument5 pages3D CD Technique Completes Dentures in 3 Days for ElderlyMaulida Dara HarjantiNo ratings yet
- Pamela Grace B. Demaisip: Employment RecordDocument4 pagesPamela Grace B. Demaisip: Employment RecordPearl Demaisip-NinonuevoNo ratings yet
- Exploring The Influence of Nursing Work Environment and PatientDocument7 pagesExploring The Influence of Nursing Work Environment and PatientNatalia Herlin KaligisNo ratings yet
- Rajiv Gandhi University of Health Sciences, Karnataka.: 4th ' T ' Block, Jayanagar, Banglore-560 041Document12 pagesRajiv Gandhi University of Health Sciences, Karnataka.: 4th ' T ' Block, Jayanagar, Banglore-560 041SunnyNo ratings yet
- The Federal Emergency Agency (FEMA)Document3 pagesThe Federal Emergency Agency (FEMA)oguturonnieNo ratings yet
- Brady Plague Inc Game PDFDocument6 pagesBrady Plague Inc Game PDFapi-348251135No ratings yet
- Ibd FormDocument2 pagesIbd FormJohn Henry100% (1)
- Table 5 Sample Family Nursing Care PlanDocument3 pagesTable 5 Sample Family Nursing Care PlanKenmiharu Soriano100% (5)
- Multi-Specialty Hospital.Document21 pagesMulti-Specialty Hospital.Sagar RavalNo ratings yet
- MR Zu KankerDocument21 pagesMR Zu KankerZudan Ady Wijaya AptNo ratings yet
- Improve Patient Safety with SBAR CommunicationDocument3 pagesImprove Patient Safety with SBAR CommunicationNikiNo ratings yet