Professional Documents
Culture Documents
Soluition Charpter 1 - 2
Uploaded by
Bryan de Barros0 ratings0% found this document useful (0 votes)
8 views1 pageJones solution Chapter 1/2
Original Title
Soluition Charpter 1_2
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentJones solution Chapter 1/2
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
8 views1 pageSoluition Charpter 1 - 2
Uploaded by
Bryan de BarrosJones solution Chapter 1/2
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
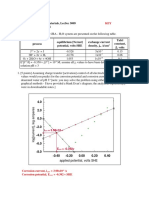
EMA 4324 Problem Set 2 KEY
1-9. A carbon steel test specimen of dimensions 2-inch by 3-inch by 0.125-inch
with a 0.25-inch diameter hole for suspending in solution is exposed for 120
hours in an acid solution and loses 150 milligrams in weight. Calculate the
corrosion rate in mpy and in microns/hour.
******************************************************************
AS = 2(2*3)+2(2*0.125)+2(3*0.125)-2*(π*0.252/4)+π*0.25*0.125
= 13.25 in2 *(2.54cm/inch)2 = 85.5 cm2
CR = (150/1000)/7.87 g/cm3/85.5 cm2 = 2.23x10-4 cm/120 hrs = 1.86x10-6
cm/hr/2.54 cm/in*1000 mil/inch*24 hr/day
*365 day/year = 6.40 mpy
1-11. Three more identical test specimens are included with the one from problem 2
in a planned interval testing program. One is exposed for 12 hours and loses
25 mg. A second is exposed for 108 hours and loses 130 mg. The third is
inserted into the solution when the second is removed and shows a weight
loss of 15 mg after removal with the specimen of problem 2. What is the
effect of time on the solution corrosiveness and on the specimen
corrodability?
*****************************************************************
comparing 25 mg loss [at start] with 15 mg [at end], [B<A1] and
corrosiveness is decreasing
comparing 150-130 = 20 with 15 [A2>B] , corrodability is increasing
You might also like
- Assignment 2 Fluid Particles and ProcessesDocument29 pagesAssignment 2 Fluid Particles and ProcessesSenanLg100% (3)
- Astm - E2180.29580Document4 pagesAstm - E2180.29580Bryan de BarrosNo ratings yet
- Kema Ageing Test 5000 Hrs.Document12 pagesKema Ageing Test 5000 Hrs.Tarun AggarwalNo ratings yet
- Advanced Temperature Measurement and Control, Second EditionFrom EverandAdvanced Temperature Measurement and Control, Second EditionNo ratings yet
- Astm D2196-10Document5 pagesAstm D2196-10Bryan de BarrosNo ratings yet
- Problems For Zero-Order Kinetics and First-Order KineticsDocument4 pagesProblems For Zero-Order Kinetics and First-Order KineticsRoger B Verosil57% (14)
- Dosages and CalculationDocument107 pagesDosages and CalculationNomi Waqas GulNo ratings yet
- ASTM D 1511 - 00 Carbon Black-Pellet Size DistributionDocument4 pagesASTM D 1511 - 00 Carbon Black-Pellet Size Distributionalin2005No ratings yet
- Property of FRP Chart 1 PDFDocument1 pageProperty of FRP Chart 1 PDFKurt FinkNo ratings yet
- Astm - D1475.12152Document4 pagesAstm - D1475.12152Bryan de BarrosNo ratings yet
- Astm - D624.6776Document9 pagesAstm - D624.6776Bryan de BarrosNo ratings yet
- Astm - D573.25993Document6 pagesAstm - D573.25993Bryan de BarrosNo ratings yet
- Turbidity RemovalDocument6 pagesTurbidity Removalapi-546041117No ratings yet
- Bernouilli ExperimentDocument6 pagesBernouilli ExperimentDasan UmaneeNo ratings yet
- Designation: C 1203 - 91 (Reapproved 2002)Document5 pagesDesignation: C 1203 - 91 (Reapproved 2002)angeljosechuquiureNo ratings yet
- D3860 Determination of Adsorptive Capacity of Activated Carbon by Aqueous Phase Isotherm TechniqueDocument4 pagesD3860 Determination of Adsorptive Capacity of Activated Carbon by Aqueous Phase Isotherm TechniqueLaura Torres ArtunduagaNo ratings yet
- Chemical Analysis of Gypsum and Gypsum Products (Metric) : Standard Test Methods ForDocument13 pagesChemical Analysis of Gypsum and Gypsum Products (Metric) : Standard Test Methods Foroscop2009No ratings yet
- Best TGA Example of Applications ElastomersDocument37 pagesBest TGA Example of Applications Elastomers28192175No ratings yet
- Accelerated Weathering Test Conditions and Procedures For Bituminous Materials (Fluorescent UV, Water Spray, and Condensation Method)Document3 pagesAccelerated Weathering Test Conditions and Procedures For Bituminous Materials (Fluorescent UV, Water Spray, and Condensation Method)Satya kaliprasad vangaraNo ratings yet
- Aggregates For ConcreteDocument18 pagesAggregates For Concreteஅம்ரு சாந்திவேலுNo ratings yet
- GM 13 PPDocument48 pagesGM 13 PPbdoeraNo ratings yet
- Performance Examination - Aggregate Abrasion TestDocument2 pagesPerformance Examination - Aggregate Abrasion TestGuillermoNo ratings yet
- After 0.5 half-lives, the percentage of the parent isotope that remains is:(1/2)^0.5 = 0.707 = 70.7%So the percentage remaining after 0.5 half-lives is 70.7Document17 pagesAfter 0.5 half-lives, the percentage of the parent isotope that remains is:(1/2)^0.5 = 0.707 = 70.7%So the percentage remaining after 0.5 half-lives is 70.7Minnie InarapmasNo ratings yet
- CVL212 Sedimentation HW QuestionsDocument3 pagesCVL212 Sedimentation HW QuestionsABHIJEET NONDANo ratings yet
- Answers Kinetics 2Document12 pagesAnswers Kinetics 2migdalr2100% (1)
- EurlSrm Observation CS2 Hydrolysis Version3 240119Document12 pagesEurlSrm Observation CS2 Hydrolysis Version3 240119Andres RestrepoNo ratings yet
- Determining Logarithmic Viscosity Number of Poly (Vinyl Chloride) (PVC) in Formulated CompoundsDocument4 pagesDetermining Logarithmic Viscosity Number of Poly (Vinyl Chloride) (PVC) in Formulated CompoundsCheolhyeon ChoNo ratings yet
- Utility water treatment SOPDocument10 pagesUtility water treatment SOPMuhammadPurnamaSugiri50% (2)
- Climatic sequence test overviewDocument20 pagesClimatic sequence test overviewLee HeonbeomNo ratings yet
- Midterms ChemDocument27 pagesMidterms ChemAndrei Dela CruzNo ratings yet
- Standard Test Method For Resistance of Plastic Films To Extraction by ChemicalsDocument3 pagesStandard Test Method For Resistance of Plastic Films To Extraction by ChemicalsVlz ClaraNo ratings yet
- 12 Astm D 6928Document7 pages12 Astm D 6928Joel BecerraNo ratings yet
- Cement Milling Performance Factors That Influence EfficiencyDocument17 pagesCement Milling Performance Factors That Influence Efficiencyshani5573No ratings yet
- D 5757 - 95 - Rdu3ntctukveDocument4 pagesD 5757 - 95 - Rdu3ntctukveDH BNo ratings yet
- Total Moisture in Coal: Standard Test Method ForDocument8 pagesTotal Moisture in Coal: Standard Test Method ForLuisana MolinaNo ratings yet
- LA Abrasion Test Method for Aggregate ResistanceDocument3 pagesLA Abrasion Test Method for Aggregate ResistanceSalih MohayaddinNo ratings yet
- Math Calculations - AhmadDocument2 pagesMath Calculations - AhmadHelenNo ratings yet
- Los Angeles Abrasion TestDocument8 pagesLos Angeles Abrasion TestAisyah Ibrahim95% (22)
- Cooling System ArticleDocument16 pagesCooling System ArticleRiski Nalendra SukmaNo ratings yet
- Determination of Crushing Value of Coarse Aggregates and 10% Fine Value TestDocument6 pagesDetermination of Crushing Value of Coarse Aggregates and 10% Fine Value TestMazharYasinNo ratings yet
- Handout 2 Min313Document10 pagesHandout 2 Min313Jake AJNo ratings yet
- D 2238 - 92 R99 - RdiymzgDocument7 pagesD 2238 - 92 R99 - Rdiymzgjamaljamal20No ratings yet
- Section 18 Design of Plastic Gears PDFDocument15 pagesSection 18 Design of Plastic Gears PDFSergio CandiottiNo ratings yet
- Lista5 Variable LoadsDocument3 pagesLista5 Variable LoadsArtur PaixãoNo ratings yet
- PET 9130-104 NewestDocument2 pagesPET 9130-104 NewestwlssagitaNo ratings yet
- 0625 w12 QP 63 PDFDocument16 pages0625 w12 QP 63 PDFshaamini_devi_5No ratings yet
- C535 - 16 - Standard Test Method For Resistance To Degradatio of Large-Size Coarse Aggregate ByAbrasion Impact in The Los Angeles MachineDocument3 pagesC535 - 16 - Standard Test Method For Resistance To Degradatio of Large-Size Coarse Aggregate ByAbrasion Impact in The Los Angeles MachineTomás Venegas Pardo100% (1)
- Bio Eng 4316/7316 Chem Eng 4316/7316 Biomass Refinery Operations II November 16, 2015Document33 pagesBio Eng 4316/7316 Chem Eng 4316/7316 Biomass Refinery Operations II November 16, 2015curioussssNo ratings yet
- Astm C123 (2012)Document3 pagesAstm C123 (2012)Stuar TencioNo ratings yet
- ESP Lesson 7 (Industrial Applications For Fabric Filters)Document24 pagesESP Lesson 7 (Industrial Applications For Fabric Filters)jkaunoNo ratings yet
- Astm C123Document3 pagesAstm C123renzoNo ratings yet
- Scotch KoteDocument4 pagesScotch KoteJavier Gomez ReyesNo ratings yet
- Typical VI or VIM SpecDocument1 pageTypical VI or VIM SpecDinh Thang LeNo ratings yet
- Sison, Marcus Ceazar - Module 1 - SurveyingDocument2 pagesSison, Marcus Ceazar - Module 1 - SurveyingMarcus Ceazar SisonNo ratings yet
- Volatile Matter in Green Petroleum Coke Quartz Crucible ProcedureDocument4 pagesVolatile Matter in Green Petroleum Coke Quartz Crucible ProcedureAli VarmazyarNo ratings yet
- C 35 - 95 r00 - Qzm1ltk1ujawDocument3 pagesC 35 - 95 r00 - Qzm1ltk1ujaw2010civ164No ratings yet
- Full Syllabus Test-02Document14 pagesFull Syllabus Test-02Arjun Satheesh KumarNo ratings yet
- Preparing Coal Samples For Analysis: Standard Method ofDocument12 pagesPreparing Coal Samples For Analysis: Standard Method ofAnonymous T02GVGzBNo ratings yet
- Chemistry 2017Document18 pagesChemistry 2017samar ahmedNo ratings yet
- Fine Particle (2.5 microns) Emissions: Regulations, Measurement, and ControlFrom EverandFine Particle (2.5 microns) Emissions: Regulations, Measurement, and ControlNo ratings yet
- Thermodynamic Degradation Science: Physics of Failure, Accelerated Testing, Fatigue, and Reliability ApplicationsFrom EverandThermodynamic Degradation Science: Physics of Failure, Accelerated Testing, Fatigue, and Reliability ApplicationsNo ratings yet
- The Spectra and Dynamics of Diatomic Molecules: Revised and Enlarged EditionFrom EverandThe Spectra and Dynamics of Diatomic Molecules: Revised and Enlarged EditionNo ratings yet
- Vulcanized Rubber and Thermoplastic Elastomers - Tension: Standard Test Methods ForDocument14 pagesVulcanized Rubber and Thermoplastic Elastomers - Tension: Standard Test Methods ForPaulo Venicio Alves Vieira100% (1)
- Astm - D4400.39339Document4 pagesAstm - D4400.39339Bryan de BarrosNo ratings yet
- Astm - D2196.12408Document5 pagesAstm - D2196.12408Bryan de BarrosNo ratings yet
- Vulcanized Rubber and Thermoplastic Elastomers - Tension: Standard Test Methods ForDocument14 pagesVulcanized Rubber and Thermoplastic Elastomers - Tension: Standard Test Methods ForPaulo Venicio Alves Vieira100% (1)
- Astm G58.25944Document8 pagesAstm G58.25944Bryan de BarrosNo ratings yet
- Astm - G168.12911Document10 pagesAstm - G168.12911Bryan de BarrosNo ratings yet
- G95-87 Standard Test MethodDocument4 pagesG95-87 Standard Test MethodandresjypNo ratings yet
- Astm - D2805.16103Document6 pagesAstm - D2805.16103Bryan de Barros100% (2)
- Astm - G8.12737Document9 pagesAstm - G8.12737Bryan de BarrosNo ratings yet
- Effect of Cathodic Potentials On The Hydrogen Embrittlement Susceptibility of 10Ni5CrMo SteelDocument15 pagesEffect of Cathodic Potentials On The Hydrogen Embrittlement Susceptibility of 10Ni5CrMo SteelBryan de BarrosNo ratings yet
- Effect of Cathodic Potentials On The SCC Behavior of E690 Steel in Simulated SeawaterDocument10 pagesEffect of Cathodic Potentials On The SCC Behavior of E690 Steel in Simulated SeawaterBryan de BarrosNo ratings yet
- Techniques To Measure Hydrogen Content in SS 304LDocument30 pagesTechniques To Measure Hydrogen Content in SS 304LBryan de BarrosNo ratings yet
- Preparing, Cleaning, and Evaluating Corrosion Test SpecimensDocument8 pagesPreparing, Cleaning, and Evaluating Corrosion Test SpecimensLê CôngNo ratings yet
- Hydrogen Permeation Barriers - Basic Requirements, Material Selection, Deposition Methods, and Quality EvaluationDocument7 pagesHydrogen Permeation Barriers - Basic Requirements, Material Selection, Deposition Methods, and Quality EvaluationBryan de BarrosNo ratings yet
- A Study of Strain and Deformation Measurement Using The Arduino Microcontroller and Strain Gauges DevicesDocument7 pagesA Study of Strain and Deformation Measurement Using The Arduino Microcontroller and Strain Gauges DevicesDavid VilcaNo ratings yet
- Atmospheric Corrosion Sensor Based On Strain MeasurementDocument21 pagesAtmospheric Corrosion Sensor Based On Strain MeasurementBryan de BarrosNo ratings yet
- Assessing Atmospheric Corrosion with Novel SensorDocument19 pagesAssessing Atmospheric Corrosion with Novel SensorBryan de BarrosNo ratings yet
- Solution Charpter 4 - 2Document5 pagesSolution Charpter 4 - 2CANDYNo ratings yet
- StrainGage MeasurementDocument37 pagesStrainGage MeasurementfifafifaNo ratings yet
- Astm - G33.34087 - 2015Document3 pagesAstm - G33.34087 - 2015Bryan de BarrosNo ratings yet
- Quiz 3 Ema 4324, Stability of Materials, Lecsec 3009 Monday, October 11, 2004Document2 pagesQuiz 3 Ema 4324, Stability of Materials, Lecsec 3009 Monday, October 11, 2004Bryan de BarrosNo ratings yet
- NI Strain Gauge TutorialDocument12 pagesNI Strain Gauge TutorialViki Hari Fitrianto100% (3)
- Soluition Charpter 2 PDFDocument3 pagesSoluition Charpter 2 PDFBryan de BarrosNo ratings yet
- Soluition Charpter 5 - 1Document3 pagesSoluition Charpter 5 - 1Bryan de BarrosNo ratings yet
- EMA 4324 Problem Set 11 Galvanic Corrosion RatesDocument4 pagesEMA 4324 Problem Set 11 Galvanic Corrosion RatesCANDYNo ratings yet