Professional Documents
Culture Documents
Quiz 3 Ema 4324, Stability of Materials, Lecsec 3009 Monday, October 11, 2004
Uploaded by
Bryan de BarrosOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Quiz 3 Ema 4324, Stability of Materials, Lecsec 3009 Monday, October 11, 2004
Uploaded by
Bryan de BarrosCopyright:
Available Formats
Quiz 3
EMA 4324, Stability of Materials, LecSec 3009 KEY
Monday, October 11, 2004
Electrochemical data for the JRA - H2O system are presented on the following table.
Tafel
equilibrium [Nernst] exchange current
process constant,
potential, volts SHE density, jo, A/cm2
β, volts
+2 - -5
J + 2e = J -0.528 5x10 0.15
+ - -7
2H + 2e = H2 -0.178 1x10 0.06
O2 + 2H2O + 4e- = 4OH- 1.053 1x10-15 0.1
o +2 +2 -6
E [J /J] = -0.350 v; [J ] = 10 M; assume all jo values to have been determined at EN
solution pH = 3
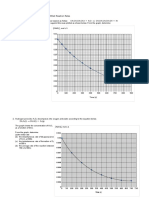
1. [5 points] Assuming charge transfer [activation] control of all electrochemical processes, what
values would you predict for the corrosion potential and corrosion current for JRA exposed to
deaerated water of pH 3? [note: you may solve the problem using either analytical or
graphical methods - I have provided a blank Evan’s diagram; the analytical expression[s]
were in downloadable notes.]
4.0
log[current], log amperes
2.0
0.0
-2.0
icorr = 3.98x10-4
-4.0
-6.0
Ecorr = -0.392v
-8.0
-0.40 -0.20 0.00 0.20 0.40
applied potential, volts SHE
Corrosion current, icorr = 3.98x10-4 A
Corrosion potential, Ecorr = -0.392 v SHE
2. [5 points] Calculate the corrosion penetration rate [CPR, in units of mpy] for JRA in the
deaerated pH3 test solution.
AW, g/mol atom radius, nm ion radius, nm density, g/cm3
0.098 [JRA+2]
JRA(c) 91.8 0.141 9.62
0.077 [JRA+3]
***************************************************************************
Had to have assume A = 1cm2 in order to use jo values - therefore, jcorr = icorr
3.98x10-4 C/s*cm2*91.8/[2*96500*9.62] = 1.97x10-8 cm/s = 245 mpy
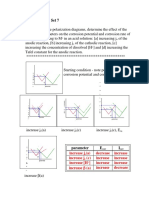
3. [10 points] For 2 points each, predict the effects of the following changes on corrosion current
and corrosion potential:
a. An increase in area of JRA exposed to the environment. Ecorr unchanged
icorr increased
[jcorr unchanged]
b. An increase in solution pH. Ecorr, icorr decrease
c. An increase in H2 pressure. no change
d. An increase in value of the Tafel constant for the
cathodic reaction [βc]. Ecorr, icorr decrease
e. Introduction of O2 [p = 1 atm]. Ecorr, icorr increase
You might also like
- Chemical Kinetics: CHEM. 204Document17 pagesChemical Kinetics: CHEM. 204myriamNo ratings yet
- Practical 4 Postlab ReportDocument8 pagesPractical 4 Postlab Reportgracebrewster123No ratings yet
- Assessment 11 WorksheetDocument3 pagesAssessment 11 WorksheetZaineb HaroonNo ratings yet
- Rates of Reaction Suroviec Spring 2014Document43 pagesRates of Reaction Suroviec Spring 2014enesffsNo ratings yet
- 2011-2012 Prequiz For Kinetics - Problems and SolutionsDocument7 pages2011-2012 Prequiz For Kinetics - Problems and SolutionsJomari GaliasNo ratings yet
- Ap-Chem Kinetics fr2Document11 pagesAp-Chem Kinetics fr2hylee102594No ratings yet
- Soluition Charpter 4 - 1Document3 pagesSoluition Charpter 4 - 1Bryan de BarrosNo ratings yet
- Homework Ed Kinetics2Document5 pagesHomework Ed Kinetics2Edrian A. MañalongNo ratings yet
- ElectrochemistryDocument12 pagesElectrochemistrydenisNo ratings yet
- 10Document4 pages10ZenPhiNo ratings yet
- Chemical KineticsDocument22 pagesChemical KineticsEleanorNo ratings yet
- Assignment 1 CY101 (Chemistry For Engineers)Document2 pagesAssignment 1 CY101 (Chemistry For Engineers)Hemant MeenaNo ratings yet
- Tugas II Fenomena Transport LanjutDocument3 pagesTugas II Fenomena Transport Lanjutزينل الغزاليNo ratings yet
- The Advanced Placement Examination in Chemistry: ElectrochemistryDocument21 pagesThe Advanced Placement Examination in Chemistry: ElectrochemistrySreeyaNo ratings yet
- Study:Screen Output: Result SummaryDocument31 pagesStudy:Screen Output: Result SummaryRamkumarNo ratings yet
- 1.1 Saturator 1.1.1 Process Description: H O From Distillation ColumnDocument20 pages1.1 Saturator 1.1.1 Process Description: H O From Distillation ColumnNUR AKMAL HISHAMNo ratings yet
- CSTR Unit Ops LabDocument7 pagesCSTR Unit Ops LabKelly Sheine SisonNo ratings yet
- A2 Extension1 Electrochemistry and RedoxDocument10 pagesA2 Extension1 Electrochemistry and RedoxDavid MathewsNo ratings yet
- Topic 3 - ElectrochemistryDocument7 pagesTopic 3 - ElectrochemistryMutasimNo ratings yet
- Assignment 7Document2 pagesAssignment 7LelyNo ratings yet
- Exercises KineticsDocument7 pagesExercises KineticsFahad AlasmiNo ratings yet
- 201-Electrochem Revised PDFDocument42 pages201-Electrochem Revised PDFAjay AjayNo ratings yet
- EG2010 Practice ExamDocument7 pagesEG2010 Practice ExamEdwin JomonNo ratings yet
- Solutions-Electrochemistry Practice ProblemsDocument6 pagesSolutions-Electrochemistry Practice ProblemsNga TranNo ratings yet
- P8-4 (Page 572 Fogler 4th Ed.) : K M V VDocument15 pagesP8-4 (Page 572 Fogler 4th Ed.) : K M V VSILPA ASTI NURANo ratings yet
- Solution EE4701 Sp18 T3Document4 pagesSolution EE4701 Sp18 T3al-muntheral-mairikiNo ratings yet
- Vegard's Law: DescriptionDocument10 pagesVegard's Law: DescriptionEzekiel FikiruNo ratings yet
- Practice Problems in Chemical Reaction Engineering For GATEDocument16 pagesPractice Problems in Chemical Reaction Engineering For GATERasNo ratings yet
- Modeling and Simulation of RLC Circuit (Band Pass Filter)Document25 pagesModeling and Simulation of RLC Circuit (Band Pass Filter)elneelNo ratings yet
- Cap 2Document33 pagesCap 2Ricardo Rincon VegaNo ratings yet
- Problem Set No. 2: Transformer Equivalent CircuitDocument3 pagesProblem Set No. 2: Transformer Equivalent CircuitGeva Garrado100% (2)
- Module 1 - Electrochemistry (Part 2)Document13 pagesModule 1 - Electrochemistry (Part 2)Steven LeeNo ratings yet
- AP Chemistry 2010 Free-Response Questions Form B: The College BoardDocument13 pagesAP Chemistry 2010 Free-Response Questions Form B: The College BoardDharul Handri PranawaNo ratings yet
- P19 Answers Albert KweyeteDocument6 pagesP19 Answers Albert KweyetedenisNo ratings yet
- 09 (2) PhysChem Exam-AnswersDocument10 pages09 (2) PhysChem Exam-Answerstiffanyyy00No ratings yet
- Exam With Solution 2023-All-FinalDocument15 pagesExam With Solution 2023-All-FinalsaraNo ratings yet
- CHE 509 - Past Exam QuestionsDocument12 pagesCHE 509 - Past Exam QuestionsJane Eilyza Aballa100% (1)
- Kinetics Worksheet AnswersDocument7 pagesKinetics Worksheet AnswerslinaNo ratings yet
- Drills For An A CHM096Document20 pagesDrills For An A CHM096Aiman FitryNo ratings yet
- Comprehensive Evaluation of Electromagnetic Heating Well Stimulation For Enhancing Production From Heavy and Extra-Heavy Oil ReservoirsDocument9 pagesComprehensive Evaluation of Electromagnetic Heating Well Stimulation For Enhancing Production From Heavy and Extra-Heavy Oil ReservoirsLeo Van GintingNo ratings yet
- Electrochem PresentationDocument44 pagesElectrochem PresentationNikitaNo ratings yet
- Chem1000 2018 & 2019 Pastpapersnm4Document19 pagesChem1000 2018 & 2019 Pastpapersnm4mulengamordecai92No ratings yet
- Department of Mining Engineering: Group MembersDocument6 pagesDepartment of Mining Engineering: Group MembersSarem AlemuNo ratings yet
- Analytical+Kinetics+QuizADocument4 pagesAnalytical+Kinetics+QuizAPatrickNo ratings yet
- Munn - Metal-Organic Frameworks SIDocument10 pagesMunn - Metal-Organic Frameworks SIjeppoo1No ratings yet
- Solutions Manual: Electric Motor DrivesDocument12 pagesSolutions Manual: Electric Motor DrivesMedo Sabah100% (2)
- Reaction Engineering I-Problem Sheet IIDocument7 pagesReaction Engineering I-Problem Sheet IISimay AydoganNo ratings yet
- Module 7 Problem Set Answer KeyDocument3 pagesModule 7 Problem Set Answer KeyPauline Grace CadusaleNo ratings yet
- Test2 SolutionDocument10 pagesTest2 SolutionHua KhienNo ratings yet
- Annex P - Allowable External Loads On Tank Shell OpeningsDocument7 pagesAnnex P - Allowable External Loads On Tank Shell OpeningsArmando VegaNo ratings yet
- Ders 14 Chemical Kinetics PDFDocument25 pagesDers 14 Chemical Kinetics PDFÖmer ErcanNo ratings yet
- Wa0031.Document40 pagesWa0031.SefalikaNo ratings yet
- Celda ElectrolíticaDocument18 pagesCelda ElectrolíticaJenniffer LineroNo ratings yet
- (A) RC 1/200 For The Resistor, V Ir : Chapter 7, Solution 1Document97 pages(A) RC 1/200 For The Resistor, V Ir : Chapter 7, Solution 1Haseeb ArifNo ratings yet
- Chapter 18 BQDocument10 pagesChapter 18 BQTarek GhaddarNo ratings yet
- Electro SulDocument4 pagesElectro SulChutvinder LanduliyaNo ratings yet
- Orca - Share - Media1575204849829 2Document113 pagesOrca - Share - Media1575204849829 2Lily Antonette AgustinNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Electrochemical Processes in Biological SystemsFrom EverandElectrochemical Processes in Biological SystemsAndrzej LewenstamNo ratings yet
- Astm - D573.25993Document6 pagesAstm - D573.25993Bryan de BarrosNo ratings yet
- Astm - G168.12911Document10 pagesAstm - G168.12911Bryan de BarrosNo ratings yet
- Astm G58.25944Document8 pagesAstm G58.25944Bryan de BarrosNo ratings yet
- Astm - D1475.12152Document4 pagesAstm - D1475.12152Bryan de BarrosNo ratings yet
- Astm - D624.6776Document9 pagesAstm - D624.6776Bryan de BarrosNo ratings yet
- Astm - D4400.39339Document4 pagesAstm - D4400.39339Bryan de BarrosNo ratings yet
- Astm - D2196.12408Document5 pagesAstm - D2196.12408Bryan de BarrosNo ratings yet
- Astm - D2805.16103Document6 pagesAstm - D2805.16103Bryan de Barros100% (2)
- Astm - E2180.29580Document4 pagesAstm - E2180.29580Bryan de BarrosNo ratings yet
- Techniques To Measure Hydrogen Content in SS 304LDocument30 pagesTechniques To Measure Hydrogen Content in SS 304LBryan de BarrosNo ratings yet
- Soluition Charpter 5 - 1Document3 pagesSoluition Charpter 5 - 1Bryan de BarrosNo ratings yet
- Soluition Charpter 1 - 2Document1 pageSoluition Charpter 1 - 2Bryan de BarrosNo ratings yet
- Soluition Charpter 3 - 3Document1 pageSoluition Charpter 3 - 3Bryan de BarrosNo ratings yet
- Soluition Charpter 3 - 2 PDFDocument3 pagesSoluition Charpter 3 - 2 PDFBryan de BarrosNo ratings yet
- Soluition Charpter 4 - 1Document3 pagesSoluition Charpter 4 - 1Bryan de BarrosNo ratings yet
- Assessing Atmospheric Corrosion of Metal by A Novel Electrochemical Sensor Combining With A Thin Insulating Net Using Electrochemical Noise TechniqueDocument19 pagesAssessing Atmospheric Corrosion of Metal by A Novel Electrochemical Sensor Combining With A Thin Insulating Net Using Electrochemical Noise TechniqueBryan de BarrosNo ratings yet
- 2-7. (Note That I Have Modified The Problem in The Textbook.) ADocument2 pages2-7. (Note That I Have Modified The Problem in The Textbook.) ABryan de BarrosNo ratings yet
- Soluition Charpter 3 - 2 PDFDocument3 pagesSoluition Charpter 3 - 2 PDFBryan de BarrosNo ratings yet
- EMA 4324 Problem Set 1Document2 pagesEMA 4324 Problem Set 1Bryan de BarrosNo ratings yet
- General Physical Properties of CO in Compression and Transportation ProcessesDocument9 pagesGeneral Physical Properties of CO in Compression and Transportation ProcessesBryan de BarrosNo ratings yet
- Tesla and Time TravelDocument3 pagesTesla and Time TravelLaron Clark50% (2)
- AP Physics II - Week of 4.20 - 4.24 ActivitiesDocument10 pagesAP Physics II - Week of 4.20 - 4.24 ActivitiesNephtali Pinos-anNo ratings yet
- Niels BohrDocument2 pagesNiels Bohrmr personalNo ratings yet
- Dielectric Phenomena in High Voltage Engineering, 2nd EdDocument326 pagesDielectric Phenomena in High Voltage Engineering, 2nd Edkgrhoads100% (7)
- The Operational Mechanism of Continuous Four-Roll Plate Bending ProcessDocument4 pagesThe Operational Mechanism of Continuous Four-Roll Plate Bending ProcessBenjamin Neciosup PaucarNo ratings yet
- Electronic and CircuitsDocument772 pagesElectronic and CircuitsIbrahim A Said67% (3)
- Zigzag Transformer - Some New Applications With A Note To Energy EfficiencyDocument15 pagesZigzag Transformer - Some New Applications With A Note To Energy EfficiencydoriaNo ratings yet
- Cam DesigningDocument26 pagesCam DesigningBilal TayyabNo ratings yet
- 01 Density PDFDocument12 pages01 Density PDFshoaib akhtarNo ratings yet
- Nurture Test Series / Joint Package Course: Distance Learning ProgrammeDocument8 pagesNurture Test Series / Joint Package Course: Distance Learning ProgrammeRebanta BeraNo ratings yet
- PICASSO SNOLAB 12a 0Document44 pagesPICASSO SNOLAB 12a 0Ivan FelisNo ratings yet
- NEHA Mechanical Properties BiomaterialsDocument19 pagesNEHA Mechanical Properties BiomaterialsRavishanker BaligaNo ratings yet
- Physics Investigatory Project Atharav SharmaDocument16 pagesPhysics Investigatory Project Atharav SharmaJatin MehtaNo ratings yet
- 11th Class PhysicsDocument73 pages11th Class PhysicsRishabh SharmaNo ratings yet
- Fisica 1 TemasDocument36 pagesFisica 1 TemasUsuario 1023No ratings yet
- Master1 AM CoursesDocument3 pagesMaster1 AM CoursesParokotil MidhunNo ratings yet
- 0625 s14 QP 22 PDFDocument16 pages0625 s14 QP 22 PDFHaider AliNo ratings yet
- The Clock of The Long Now PDFDocument325 pagesThe Clock of The Long Now PDFpaulaisabel2No ratings yet
- (4104) DPP 32 50 B PDFDocument109 pages(4104) DPP 32 50 B PDFRAJDEEP DASNo ratings yet
- Layout Solns 3Document12 pagesLayout Solns 3VIKRAM KUMARNo ratings yet
- A Power Presizing Methodology For Electric Vehicle Traction MotorsDocument9 pagesA Power Presizing Methodology For Electric Vehicle Traction MotorsGanesan TNo ratings yet
- Presentation On TransformerDocument15 pagesPresentation On TransformerManish RanaNo ratings yet
- Body of Knowledge (Science)Document5 pagesBody of Knowledge (Science)Putri HandayaniNo ratings yet
- Barona, Ailene N. Iecep-Manila Test Questions. GeasDocument12 pagesBarona, Ailene N. Iecep-Manila Test Questions. GeasJmae BantilingNo ratings yet
- Rancangan Pengajaran Dan Pembelajaran Harian Minggu 1: Perhimpunan Rasmi Di Dataran BestariDocument11 pagesRancangan Pengajaran Dan Pembelajaran Harian Minggu 1: Perhimpunan Rasmi Di Dataran BestarimalaomarNo ratings yet
- Boiler Perform CalculateDocument29 pagesBoiler Perform CalculateAnsarNo ratings yet
- DSM Vibration Welding PDFDocument20 pagesDSM Vibration Welding PDFraj202987_47312067100% (1)
- Physical Chemistry Chapter 8 LaidlerDocument46 pagesPhysical Chemistry Chapter 8 LaidlerCody Ewell0% (1)
- Solid State Physics JEST 2012-2016Document7 pagesSolid State Physics JEST 2012-2016Puneet SharmaNo ratings yet