Professional Documents
Culture Documents
X Sanskrit SQP 2018 19
Uploaded by
Sameer SinhaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
X Sanskrit SQP 2018 19
Uploaded by
Sameer SinhaCopyright:
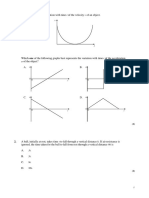
Available Formats
12th
Read the following instructions carefully before you begin to answer the questions in the OMR Answer Sheets provided
along with this question paper. Ask the Examination In-charge/Invigilator how to mark the OMR Answer Sheets, in case you
have any doubts.
INSTRUCTION TO CANDIDATES TO BE EXPLAINED BY THE INVIGILATORS
1. This Booklet contains 40 questions. All questions carry an equal marks of 2.5 each.
2. All questions are compulsory.
3. The paper is divided into 4 sections. Section A and B is compulsory for all the candidates. However section C
and D is to be answered by the candidates as per their choice of subject, i.e. either Mathematics or Biology.
4. This Booklet contains 6 pages. Please check, if any page is misprintied, missing or repeated.
5. Collect your OMR Answer Sheets from the Invigilator/Examination In-Charge to answer the questions given in
this Booklet.
6. You must fill all the necessary information's which are required in the space provided in this booklet and OMR
Answer Sheet.
7. Correct Answers must be marked by “Darkening” the corresponding circles on the OMR Answer Sheet, against
the relevant question number with Pencil or Blue/Black, Ball point Pen only. Answers which are not darkened in
circle will not be awarded with any mark.
8. Space for rough work is provided in this booklet. No rough work is to be done in the OMR Answer Sheet.
9. Mobile Phones and other Wireless equipments are banned in the examination halls/rooms.
10. OMR Answer Sheets must be handed over to the Examination In-charge/Invigilator before you leave the
examination room/hall and recheck that you have filled all the required informations.
11. The results will be published in our web-site WWW.SILVERZONE.ORG in the month of Jan 2014. You can check
it with your 12 digit Roll Number provided in the Enrollment Ticket.
TEACHERS ARE REQUESTED TO CHECK IF THE REQUIRED INFORMATIONS ARE PROPERLY FILLED BY THE CANDIDATES IN THE
QUESTION PAPER & OMR ANSWER SHEETS, AND ALSO ENSURE THAT OMR ANSWER SHEETS ARE PROPERLY MARKED.
PLEASE SEND US BACK THE OMR ANSWER SHEETS ONLY.
Note: Return this question
paper along with answer sheet
REF/iOS13/DD | www.silverzone.org | Class 12 - Page : 1
SECTION - A : PHYSICS
1. A metallic sphere A of radius r 1 carries a 5. In the circuit shown in the following figure,
charge Q. It is brought in contact with an the equivalent resistance between points
uncharged sphere B of radius r2. The charge A and B is:
on sphare A now will be
r1 Q r2 Q
(A) r2
(B) r1
r2 Q r1 Q
(C) r1 + r2
(D) r1 + r2
3R 5R
(E) None of these (A) (B)
4 6
2. An electric dipole placed with its axis
7R 11R
inclined at an angle to the direction of a (C) (D)
uniform electric field experiences: 10 9
(A) A force but no torque (E) None of these
(B) A torque but no force 6. A transformer is used for
(C) A force as well as a torque (A) Converting mechanical energy into
(D) Neither a force nor a torque electrical energy
(E) None of these (B) Increasing or decreasing a d.c. voltage
3. Three charges, each of magnitude q = 2 µ C (C) Converting an a.c. voltage into a d.c. voltage
(D) Increasing or decreasing an a.c. voltage
are placed at the vertices, P, Q and R of the
(E) None of these
triangle as shown in figure given below. The
sum of the sides PQ and PR is 12 cm and their 7. A carbon nucleus emits a particle x and changes
product is 32 cm2. The potential at point P into nitrogen according to the equation
would be 14 C 14 N + x
6 7
What is x?
(A) An electron (B) A proton
(C) An alpha particle (D) A photon
(E) None of these
(A) 6.00 × 105 V (B) 6.25 × 105 V
(C) 6.50 × 105 V (D) 6.75 × 105 V 8. A current carrying wire AB is placed near
(E) None of these another CD as shown in figure. Wire CD is
fixed while wire AB is free to move. When a
4. The following figure shows a network of
current is passed through wire AB, it will have
capacitors where C1 = C2 = C3 = C4 = 4 µ F and
C 5 = 5 µ F. The equivalent capacitance
between points A and B is:
(A) Only translational motion
(B) Only rotational motion
(A) 4µ F (B) 5 µ F
(C) Both translational as well as rotational motions
(C) 16 µ F (D) 20 µ F (D) Neither translational nor rotational motion
(E) None of these (E) None of these
CLASS 12 - PAGE : 2 | SILVERZONE - THE BIGGEST INTERNATIONAL OLYMPIADS | REF/iOS13/DD
9. A coil of metal wire is kept stationary in a 13. The magnetic field at the nucleus of a
non-uniform magnetic field, hydrogen atom due to the motion of an
(A) An emf and current are both induced in electron in the n th orbit is inversely
the coil proportional to:
(B) A current but no emf is induced in the coil
(A) n 2 (B) n 3
(C) An emf but no current is induced in the coil 4
(D) Neither emf nor current is induced in (C) n (D) n 5
the coil (E) None of these
(E) None of these 14. Alpha particles are fired at a nucleus. Which
10. A lens of power + 2.0 D is placed in contact of the paths shown in figure is not possible?
with another lens of power – 1.0 D. The
combination will behave like
(A) A converging lens of focal length 100 cm
(B) A diverging lens of focal length 100 cm
(C) A converging lens of focal length 50 cm
(D) A diverging lens of focal length 50 cm
(E) None of these
11. When a glass prism of refracting angle 60° is (A) 1 (B) 2
immersed in a liquid, its angle of minimum (C) 3 (D) 4
(E) None of these
deviation is 30°. The critical angle of glass
with respect to the liquid medium is: 15. Choose the wrong statement. A thermonuclear
(A) 42° (B) 45° fusion reactor is better than a fission reactor
(C) 50° (D) 52° for the following reasons:
(E) None of these (A) For the same mass of substances involved,
12. Monochromatic light of wavelength λ in air a fusion reaction releases much more
energy than a fission reaction
is refracted into a glass slab of refractive
(B) A fusion reaction can be much more easily
index µ . The wavelength of light in glass is: controlled than a fission reaction
(A) λ (B) μ λ (C) A fusion reaction produces almost no
radioactive waste
λ λ
(C) µ (D) (D) The fuel required for fusion is readily
µ2 available in abundance from sea-water
(E) None of these (E) None of these
SECTION - B : CHEMISTRY
(C) It is equal to the molecularity of an
16. In the chemical reaction, CaCO3(s)
CaO(s) elementary reaction
+ CO2(g), the pressure of CO2(g) depends on: (D) It is sum of the powers of concentration
(A) The mass of CaCO3(s) terms in the differential rate law of a reaction
(B) Temperature of the system (E) None of these
(C) The masses of both CaCO3(s) and CaO(s)
18. An isotope of the parent element is
(D) The mass of CaO(s)
(E) None of these produced with the emission of:
(A) One α - and one β -particle
17. Which of the following statements is
(B) One α - and two β -particles
incorrect about order of a reaction?
(C) Two α - and one β -particless
(A) Order of a reaction can never be equal
to zero or fractional value (D) Two α - and two
o β -particless
(B) It is always determined experimentally (E) None of these
REF/iOS13/DD | www.silverzone.org | Class 12 - Page : 3
19. The chemical processes in the production (D) The normality of the solution
of steel from haematite ore involve (E) None of these
(A) Reduction 26. The rate constant of a reaction depends on
(B) Oxidation (A) Temperature
(C) Reduction followed by oxidation (B) Initial concentration of the reactants
(D) Oxidation followed by reduction (C) Time of reaction
(E) None of these (D) Extent of reaction
20. Which of the following is expected to have (E) None of these
the lowest boiling point? 27. Physical adsorption
(A) CH3CH 2OH (B) CH3CHO (A) Involves the weak attractive interactions
(C) CH3COOH (D) CH 3OCH 3 between the adsorbent and adsorbate
(E) None of these (B) Involves the chemical interactions
21. A new carbon-carbon bond formation is between the adsorbent and adsorbate
possible in: (C) Is irreversible in nature
(A) Cannizzaro reaction (D) Increases with increase in temperature
(B) Friedel-Carfts alkylation (E) None of these
(C) Reimer-Tiemann reaction 28. A colloidal solution can be purified
(D) Both (B) and (C) following the method of
(E) None of these (A) Dialysis
22. Which of the following reagents can convert (B) Peptization
benzenediazonium chloride into benzene? (C) Filtration
(A) Water (D) Oxidation
(B) Acid (E) None of these
(C) Hypophosphorous acid
29. Starch is a polymer of:
(D) HCI
(A) Glucose
(E) None of these
(B) Fructose
23. The molecular formula of Glauber’s salt is:
(C) Sucrose
(A) MgSO4 . 7H2O
(D) Ribose
(B) Na2O2. 5H2O
(E) None of these
(C) FeSO4 . 7H2O
(D) Na2SO4 . 10H2O 30. The correct acidity order of the compounds
(E) None of these
24. The half-life of a radioactive isotope is 3 h.
What mass out of 100 g is left after 15 h?
(A) 12.5 g (B) 6.25 g
(C) 3.125 g (D) 1.562 g
(E) None of these
25. The vapour-pressure lowering of a solvent (A) (III) > (IV) > (II) > (I)
is proportional to (B) (IV) > (III) > (I) > (II)
(A) The mole fraction of the solute (C) (III) > (II) > (I) > (IV)
(B) The mole fraction of the solvent (D) (II) > (III) > (IV) > (I)
(C) The molality of the solvent (E) None of these
CLASS 12 - PAGE : 4 | SILVERZONE - THE BIGGEST INTERNATIONAL OLYMPIADS | REF/iOS13/DD
SECTION - C : MATHEMATICS
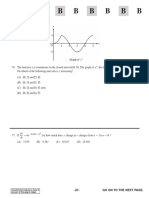
31. Consider the following relation: 36. 2
x sin xdx =
(1) A - B = A - (A B)
x2 x 1
(2) A = (A B) (A-B) (A) + sin2x + cos2x + C
4 4 8
(3) A - (B C) = (A - B) (A - C)
Which of these is/are correct: x2 x 1
(B) – sin2x + cos2x + C
(A) 1 and 3 (B) 2 only 4 4 8
(C) 2 and 3 (D) 1 and 2
(E) None of these x2 x 1
(C) + sin2x – cos2x + C
-1 5π 4 4 8
32. The value of {sin (sin )} is:
6
π 2π x2 x 1
(A) (B) (D) – sin2x – cos2x + C
6 3 4 4 8
(E) None of these
5π 6π
(C) (D)
6 3 2π x
37. 1 + sin dx =
(E) None of these 0 2
33. If A and B are two matrices such that AB = B (A) 0 (B) 2
and BA = A, then A 2 + B2 is equal to (C) 8 (D) 4
(A) A + B (B) 2 BA (E) None of these
(C) 2 AB (D) AB
2
(E) None of these dy
38. If y = A cos nx + B sin nx, than 2
=
dy ex + e x dx
34. Find , when y =
dx x x (A) -n 2 y (B) -y
e e
(C) n 2 y (D) x
8 2 (E) None of these
2 2
(A) ex ex
(B)
ex + e x 39. If the events A and B are mutually exclusive,
A
4 2 than P =
2 2 B
(C) ex ex (D) ex ex
P(A B)
(E) None of these (A) (B) 1
P(B)
35. The equation of the normal to the curve y =
P(A B)
πx (C) (D) 0
sin at (1, 1) is: P(A)
2
(E) None of these
(A) x = 1 (B) y = 1
40. If nP5 = 9 × n-1P4, than the value of n is:
2 (A) 6 (B) 8
(C) y = x (D) y - 1 =
π (C) 9 (D) 5
(E) None of these (E) None of these
REF/iOS13/DD | www.silverzone.org | Class 12 - Page : 5
SECTION - D : BIOLOGY
31. Fusion of dissimilar gametes is known as: 37. If the DNA codons are ATG ATG ATG and a
(A) Fertilization (B) Dichogamy cytosine base is inserted at the beginning
(C) Autogamy (D) Allogamy then which one of the following will result
(E) None of these in m-RNA?
32. Fertilization in earthworm occurs in: (A) CATG ATG ATG (B) CA TGA TGA TG
(A) Cocoon (B) Spermathecae (C) CAT GAT GATG (D) GUA CUA CUAC
(C) Coelom (D) Seminal vesicles (E) None of these
(E) None of these
38. The exaggerated response of the immune
33. What do you mean by the term system to certain antigens present in the
spermitogenesis? environment is called:
(A) Conversion of spermatids to sperm (A) Allergy
(B) Conversion of spermogonium to spermatid (B) Immunity
(C) Conversion of spermatid to spermogonium (C) Auto-immune
(D) Conversion of primary spermatocyte to
(D) Passive immunization
secondary spermatocyte
(E) None of these (E) None of these
34. Tunica albuginea is the covering of: 39. Transformation of normal cells into
(A) Liver (B) Spleen cancerous neoplastic cells may be induced
(C) Testis (D) Lungs by:
(E) None of these (A) Physical agent
35. The transfer of genetic material from one (B) Chemical agent
bacterium to another by virus is called: (C) Biological agent
(A) Transduction (B) Translation (D) All of these
(C) Conjugation (D) Transformation (E) None of these
(E) None of these 40. Individual of a species which occurs in a
36. The maximum formation of m-RNA occurs in: particular area constitute:
(A) Cytoplasm (B) Nucleolus (A) Flora (B) Fauna
(C) Ribosome (D) Nucleoplasm (C) Population (D) Flora and fauna
(E) None of these (E) None of these
Space for Rough Work
CLASS 12 - PAGE : 6 | SILVERZONE - THE BIGGEST INTERNATIONAL OLYMPIADS | REF/iOS13/DD
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Transport Phenomena ProblemsDocument26 pagesTransport Phenomena ProblemsCamilo SierraNo ratings yet
- Newton's Experiments On LightDocument5 pagesNewton's Experiments On LightShauna Prettygyol HamilNo ratings yet
- DPM FluentDocument182 pagesDPM Fluentபார்த்தசாரதி சுப்ரமணியன்No ratings yet
- CHEM10003 - Mock Exam 1Document17 pagesCHEM10003 - Mock Exam 1Sunny XiaNo ratings yet
- Ib Physics 2024 ChecklistDocument29 pagesIb Physics 2024 Checklistellie du123No ratings yet
- Clutches and BrakesDocument10 pagesClutches and BrakesKhaloud Mahdi Al-JasmiNo ratings yet
- Proceedings of Spie: Design and Simulation Analysis of A Magnetic Shielding Box For Ring Laser GyroscopeDocument9 pagesProceedings of Spie: Design and Simulation Analysis of A Magnetic Shielding Box For Ring Laser GyroscopeTanzil ZaidiNo ratings yet
- Physical Properties of Soil PDFDocument16 pagesPhysical Properties of Soil PDFAshish MittalNo ratings yet
- Physics Notes For Competitive Exam PDFDocument150 pagesPhysics Notes For Competitive Exam PDFAnonymous ekYPeuO100% (1)
- 4 TH BatchDocument37 pages4 TH Batchanil kumarNo ratings yet
- JurnalDocument96 pagesJurnalDheva NiaNo ratings yet
- Light by Gs MonkDocument470 pagesLight by Gs MonkSibani Sarojini100% (1)
- Revision Test PhysicsDocument56 pagesRevision Test PhysicsAkshay Khanzode100% (4)
- Test 21kinematics20151002Document26 pagesTest 21kinematics20151002laguiiniNo ratings yet
- Statement of Teaching Interests-Example 3Document2 pagesStatement of Teaching Interests-Example 3NedelcuGeorgeNo ratings yet
- Calibration of High Voltage Resistor DividersDocument2 pagesCalibration of High Voltage Resistor DividersLina Muñoz CantilloNo ratings yet
- MBC21 JNotesDocument77 pagesMBC21 JNotesAbisai Maringe AbbieNo ratings yet
- Optical Properties of SemiconductorsDocument66 pagesOptical Properties of SemiconductorsCarolina UntilaNo ratings yet
- Fin Plate Beam-To-column-flange Connection (GB)Document16 pagesFin Plate Beam-To-column-flange Connection (GB)Vlad MosNo ratings yet
- Redox Practice Quiz 11Document2 pagesRedox Practice Quiz 11rk_gummaluri5334100% (1)
- Table of Integrals PDFDocument1 pageTable of Integrals PDFDytchemNo ratings yet
- Effect of Segregation Bands On Corrosion of Steel Plate For Ship Hull PDFDocument6 pagesEffect of Segregation Bands On Corrosion of Steel Plate For Ship Hull PDFMuchamadAsyhariNo ratings yet
- Lecture 03Document25 pagesLecture 03Magdalena SiahaanNo ratings yet
- Bus BarsDocument36 pagesBus Barstceterex100% (1)
- Problem Set 6 4Document4 pagesProblem Set 6 4SowmitraDasNo ratings yet
- Flow Properties Testing and Powder Flowability - Powder & Bulk Solids Solutions PDFDocument3 pagesFlow Properties Testing and Powder Flowability - Powder & Bulk Solids Solutions PDFSukaran SinghNo ratings yet
- States of Matter-I Gas: Course OutlineDocument9 pagesStates of Matter-I Gas: Course OutlineMansoor SarwarNo ratings yet
- Abhyudaya - Theme WriteupsDocument6 pagesAbhyudaya - Theme WriteupsJuhi SinghNo ratings yet
- BC YES Calculator MCDocument10 pagesBC YES Calculator MCbingfang wuNo ratings yet
- Deposit Control PMsDocument18 pagesDeposit Control PMsMünir KarıncaoğluNo ratings yet