Professional Documents
Culture Documents
2020 SampleRecommendation
Uploaded by
Dauda BabaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
2020 SampleRecommendation
Uploaded by
Dauda BabaCopyright:
Available Formats
University of Maiduguri
Chemical Engineering Department
CLASS PROJECT
Design a plant to produce 40,000 metric tons (tonnes)/year of 2-ethylhexanol from
propylene and synthesis gas, assuming an operating period of 8,000 hours on
stream.
The Process
The first stage of the process is a hydroformylation (oxo) reaction from which the
main product is n-butyraldehyde. The feeds to this reactor are synthesis gas
(CO/H2 mixture) and propylene in the molar ratio 2/1, and the recycled products of
isobutyraldehyde cracking. The reactor operates at 130oC and 350 bar, using
cobalt carbonyl as catalyst in solution. The main reaction products are n- and
isobutyraldehyde in the ratio of 4:1, the former being the required product for
subsequent conversion to 2-ethylhexanol. In addition, 3% of the propylene feed is
converted to propane while some does not react.
Within the reactor, however, 6% of the n-butyraldehyde product is reduced to n-
butanol, 4% of the isobutyraldehyde product is reduced to isobutanol, and other
reactions occur to a small extent yielding high molecular weight compounds
(heavy ends) to the extent of 1% by weight of the butyraldehyde/butanol mixture
at the reactor exit.
The reactor is followed by a gas-liquid separator operating at 30 bar from which
the liquid phase is heated with steam to decompose the catalyst for recovery of
cobalt by filtration. A second gas-liquid separator operating at atmospheric
pressure subsequently yields a liquid phase of aldehydes, alcohols, heavy ends,
and water, which is free from propane, propylene, carbon monoxide, and
hydrogen.
This mixture then passes to a distillation column which gives a top product of
mixed butyraldehydes, followed by a second column which separates the two
butyraldehydes into an isobutyraldehyde stream containing 1.3% mole n-
butyraldehyde and an n-butyraldehyde stream containing 1.2% mole
isobutyraldehyde.
A cracker converts isobutyraldehyde at a pass yield of 80% back to propylene,
carbon monoxide, and hydrogen by passage over a catalyst with steam. After
separation of the water and unreacted isobutyraldehyde the cracked gas is recycled
to the hydroformylation reactor. The isobutyraldehyde is recycled to the cracker
inlet. The operating conditions of the cracker are 275oC and 1 bar.
The n-butyraldehyde is treated with a 2% w/w aqueous sodium hydroxide and
undergoes an aldol condensation at a conversion efficiency of 90%. The product
CHE 409: Principle of Plant Design
By: Engr. Dauda Baba Page 1
of this reaction, 2-ethylhexanol, is separated and then reduced to 2-ethylhexanol
by hydrogen in the presence of a Raney nickel catalyst with a 99% conversion
rate. In subsequent stages of the process (details of which are not required), 99.8%
of the 2-ethylhexanol is recovered at a purity of 99% by weight.
Feed Specifications
i. Propylene feed: 93% propylene, balance propane.
ii. Synthesis gas: from heavy fuel oil, after removal of sulfur compounds and
carbon dioxide:
H2 48.6%; CO 49.5%; CH4 0.4%; N2 1.5%.
Utilities
i. Dry saturated steam at 35 bar.
ii. Cooling water at 20oC.
iii. 2% w/w aqueous sodium hydroxide solution.
iv. Hydrogen gas: H2 98.8%; CH4 1.2%.
Scope of Design Work Required
1. Process Design Task
a. Prepare a process diagram for the plant showing the major items of equipment.
b. Prepare a material balance for the complete process.
c. Indicate the operating temperatures and pressure on process diagram in (a)
d. Tabulate the material stream indicate (indicating flow, temperature and pressure)
e. Prepare energy balances for the hydroformylation reactor and for the
isobutyraldehyde cracking reactor.
CHE 409: Principle of Plant Design
By: Engr. Dauda Baba Page 2
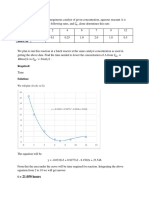
where x and y are the mole fractions of the more volatile component (isobutyraldehyde)
in the liquid and vapor phases, respectively.
CHE 409: Principle of Plant Design
By: Engr. Dauda Baba Page 3
You might also like
- Mini Project StyereneDocument25 pagesMini Project StyereneMard Apik100% (1)
- Acetaldehyde Methods 2520of 2520 ProductionDocument6 pagesAcetaldehyde Methods 2520of 2520 Productionapi-3714811100% (3)
- Design of An Plant For Manufacturing of AcetaldehydeDocument4 pagesDesign of An Plant For Manufacturing of AcetaldehydeClaudio Martinez BernalNo ratings yet
- Exhanger Leakages in VDU - ModifiedDocument14 pagesExhanger Leakages in VDU - ModifiedJay LawsonNo ratings yet
- STOICHIOMETRY (Yield, Conversion, Selectivity)Document4 pagesSTOICHIOMETRY (Yield, Conversion, Selectivity)kennethmsoriano67% (3)
- PP311 DistillationDocument173 pagesPP311 DistillationgreatnaksNo ratings yet
- Allyl Chloride OptimizationDocument37 pagesAllyl Chloride Optimizationmoheed100% (1)
- CRYOGENICS GrindingDocument18 pagesCRYOGENICS GrindingAditya AggarwalNo ratings yet
- Hydrogen Production ProcessesDocument73 pagesHydrogen Production ProcessesThitikorn Wassanarpheernphong100% (2)
- Batch Manufacture of Propylene GlycolDocument6 pagesBatch Manufacture of Propylene Glycolprassna_kamat1573No ratings yet
- TOURTON-páginas-37-124-73-88 PDFDocument16 pagesTOURTON-páginas-37-124-73-88 PDFJesús David González CañasNo ratings yet
- Hydrogen ProductionDocument26 pagesHydrogen Productionsorincarmen88No ratings yet
- Major Engineering ProblemsDocument5 pagesMajor Engineering ProblemsaathiraNo ratings yet
- Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and PowerFrom EverandThermochemical Processing of Biomass: Conversion into Fuels, Chemicals and PowerNo ratings yet
- Flowchart For AcetaldehydeDocument2 pagesFlowchart For Acetaldehydeyigitilgaz0% (1)
- Oxo SynthesisDocument1 pageOxo Synthesisdlr1233No ratings yet
- Arrieta Ethylene GlycolDocument8 pagesArrieta Ethylene GlycolNguyen VietNo ratings yet
- 2017CHE008 DetailedProcessDescription1Document17 pages2017CHE008 DetailedProcessDescription1Xi Liinett AqkoNo ratings yet
- Production of Ethylene GlycolDocument15 pagesProduction of Ethylene Glycolindahazhr100% (6)
- Kinetics (Gjjkkkgty)Document5 pagesKinetics (Gjjkkkgty)Chrysler Kane DepnagNo ratings yet
- Technological Institute of The Philippines: 363 P. Casal ST., Quiapo, ManilaDocument10 pagesTechnological Institute of The Philippines: 363 P. Casal ST., Quiapo, ManilaJohannah Jane Abuel0% (1)
- Produccion de Acido Acetico A Partir de La Oxidacion de EtilenoDocument6 pagesProduccion de Acido Acetico A Partir de La Oxidacion de EtilenoBrayan CortésNo ratings yet
- Steam Reforming Via Gas Shift, Pre Reformer and Reformer TechnologyDocument20 pagesSteam Reforming Via Gas Shift, Pre Reformer and Reformer TechnologyShawn ChanNo ratings yet
- Hydrogen Production TechnologiesFrom EverandHydrogen Production TechnologiesMehmet SankirNo ratings yet
- SRT TechnologyDocument2 pagesSRT Technologyspemo100% (3)
- Production of Methyl Ethyl Ketone From Secondary ButanolDocument2 pagesProduction of Methyl Ethyl Ketone From Secondary Butanolammadjee85% (20)
- Literature Survey On 2EH ProductionDocument3 pagesLiterature Survey On 2EH Productionkilldeadman100% (2)
- Ethylhexanol From Propylene and Synthesis GasDocument4 pagesEthylhexanol From Propylene and Synthesis GasJohn CallousNo ratings yet
- 2 Ethyl 2520hexanol Methods 2520of 2520 ProductionDocument10 pages2 Ethyl 2520hexanol Methods 2520of 2520 Productionapi-3714811No ratings yet
- Checal 2 Module ProblemsDocument3 pagesChecal 2 Module Problems5zywgtdkw5No ratings yet
- For Hysys UsersDocument5 pagesFor Hysys UsersZohaib RanaNo ratings yet
- Design (Ch.1 Problems)Document5 pagesDesign (Ch.1 Problems)John UnkNo ratings yet
- Dlawar 3Document30 pagesDlawar 3Aram Nasih MuhammadNo ratings yet
- Oxo Process for 2-Ethylhexanoic Acid ProductionDocument10 pagesOxo Process for 2-Ethylhexanoic Acid ProductionJacky WongNo ratings yet
- ManuscriptDocument18 pagesManuscriptNguyen TrangNo ratings yet
- CHE334 2023 2024 Test1Document1 pageCHE334 2023 2024 Test1peter-albert.danielNo ratings yet
- 1 s2.0 S1226086X12001566 MainDocument7 pages1 s2.0 S1226086X12001566 Mainrizanda93No ratings yet
- Batch Manufacture of Propylene GlycolDocument24 pagesBatch Manufacture of Propylene Glycoloption2010100% (1)
- Ethanol 13 AksodkopasdasmlkasmczczxcDocument10 pagesEthanol 13 AksodkopasdasmlkasmczczxcNiko Ava DaaNo ratings yet
- Simulation Lab Problem-1: Chem 2002 - Process Systems Analysis - 2016-2017Document5 pagesSimulation Lab Problem-1: Chem 2002 - Process Systems Analysis - 2016-2017ajali1957No ratings yet
- Plant Design ProjectDocument8 pagesPlant Design ProjectAbhishek SagarNo ratings yet
- Problem Set-II - Single and Multiple Units Reacting SystemDocument4 pagesProblem Set-II - Single and Multiple Units Reacting SystemDeepak TholiaNo ratings yet
- A New Method of Low-Temperature Methanol SynthesisDocument4 pagesA New Method of Low-Temperature Methanol SynthesisCarlos Alfonzo Calderón RiveroNo ratings yet
- Acrylic 2520acid Methods 2520of 2520 ProductionDocument8 pagesAcrylic 2520acid Methods 2520of 2520 Productionapi-3714811No ratings yet
- Problems in Material BalanceDocument28 pagesProblems in Material BalanceKent GardoseNo ratings yet
- Phenol Synthesis via Cumene Hydroperoxide Cleavage (Hock MethodDocument10 pagesPhenol Synthesis via Cumene Hydroperoxide Cleavage (Hock MethodRizkyanto NugrohoNo ratings yet
- CHAPTER 2 Manuf.Document9 pagesCHAPTER 2 Manuf.Sapna RanaNo ratings yet
- Chapter - 2 Process DescriptionDocument11 pagesChapter - 2 Process DescriptionSomak SahujiNo ratings yet
- Production of Ethylene Glycol: Processes and SpecificationsDocument5 pagesProduction of Ethylene Glycol: Processes and SpecificationsjorgchanNo ratings yet
- Chapter 4Document26 pagesChapter 4indumathijayakaranNo ratings yet
- CLL703 Process Engineering - Tut Sheet 1: C H C H + H C H (1/2) C H + CHDocument2 pagesCLL703 Process Engineering - Tut Sheet 1: C H C H + H C H (1/2) C H + CHshakshiNo ratings yet
- SKKK1113 Tutorial Assignment-04-ReactiveDocument2 pagesSKKK1113 Tutorial Assignment-04-ReactiveNUREEN DAYANA BINTI MOHD IZMANIZAN A21ET0194No ratings yet
- CHE 304 Optional Homework Due Toluene HyDocument9 pagesCHE 304 Optional Homework Due Toluene Hyqurat ul ainNo ratings yet
- MEK - Methods 2520of 2520productionDocument4 pagesMEK - Methods 2520of 2520productionJob MateusNo ratings yet
- Petroleum Refinery Hydrogen Production Unit ExergyDocument8 pagesPetroleum Refinery Hydrogen Production Unit ExergylovegrkNo ratings yet
- Benzene Production via Toluene HydrodealkylationDocument44 pagesBenzene Production via Toluene HydrodealkylationJudebarb94No ratings yet
- Inui 2002Document9 pagesInui 2002Rohit BabelNo ratings yet
- Production of Hydrogen by Steam Reforming of Ethanol Over A Ni/Zno CatalystDocument6 pagesProduction of Hydrogen by Steam Reforming of Ethanol Over A Ni/Zno Catalystpetro121No ratings yet
- Final ReportDocument17 pagesFinal ReportFahad KhokharNo ratings yet
- Ghita Danuta 1 16Document5 pagesGhita Danuta 1 16Anonymous p52JDZOdNo ratings yet
- Flowsheeting Sheet (1) 21/2/2015: Ag-CatalystDocument5 pagesFlowsheeting Sheet (1) 21/2/2015: Ag-CatalystAhmed Hamdy Khattab100% (1)
- 64788Document35 pages64788ghatak2100% (1)
- Synthetic Natural Gas: From Coal, Dry Biomass, and Power-to-Gas ApplicationsFrom EverandSynthetic Natural Gas: From Coal, Dry Biomass, and Power-to-Gas ApplicationsTilman J. SchildhauerNo ratings yet
- Nanoporous Catalysts for Biomass ConversionFrom EverandNanoporous Catalysts for Biomass ConversionFeng-Shou XiaoNo ratings yet
- Bio-Based SolventsFrom EverandBio-Based SolventsFrançois JérômeNo ratings yet
- AA General Product BrochureDocument6 pagesAA General Product BrochureDauda BabaNo ratings yet
- PDFDocument1 pagePDFTakanoah Ibabao EndoNo ratings yet
- Determination of Sustainable Energy Mix To Meet Nigeria Vision 20:2020 On Power Using Linear ProgrammingDocument7 pagesDetermination of Sustainable Energy Mix To Meet Nigeria Vision 20:2020 On Power Using Linear ProgrammingAZOJETENo ratings yet
- Heat ExchangersDocument48 pagesHeat ExchangersRiccat Shio'TangNo ratings yet
- ChemDocument12 pagesChemPradeep MishraNo ratings yet
- Al-Bulushi Et Al Neural Comput and Applic 2012Document13 pagesAl-Bulushi Et Al Neural Comput and Applic 2012Dauda BabaNo ratings yet
- 6103 13166 1 SM PDFDocument5 pages6103 13166 1 SM PDFDauda BabaNo ratings yet
- 6103 13166 1 SMDocument19 pages6103 13166 1 SMDauda BabaNo ratings yet
- MS Access 2013Document26 pagesMS Access 2013sreekanthNo ratings yet
- AA General Product Brochure PDFDocument28 pagesAA General Product Brochure PDFDauda BabaNo ratings yet
- Oil Production Predicting With Modified BP Neural Network MethodDocument3 pagesOil Production Predicting With Modified BP Neural Network MethodDauda BabaNo ratings yet
- C Co Certif Ompl Ficat Pletio Te On: Thi Sistoc Ertify TH HatDocument1 pageC Co Certif Ompl Ficat Pletio Te On: Thi Sistoc Ertify TH HatDauda BabaNo ratings yet
- Editorial Aifa JPSE 2014Document15 pagesEditorial Aifa JPSE 2014Dauda BabaNo ratings yet
- UP PHD Commonwealth Award - 2018 - FinalDocument3 pagesUP PHD Commonwealth Award - 2018 - FinalDauda BabaNo ratings yet
- 2018 Application FormDocument1 page2018 Application FormDauda BabaNo ratings yet
- UP PHD Commonwealth Award - 2018 - FinalDocument3 pagesUP PHD Commonwealth Award - 2018 - FinalDauda BabaNo ratings yet
- GRS CV Template 03-06-162Document3 pagesGRS CV Template 03-06-162Dauda BabaNo ratings yet
- Production Forecasting of Petroleum Reservoir Applying Higher-Order Neural Networks (HONN) With Limited Reservoir DataDocument13 pagesProduction Forecasting of Petroleum Reservoir Applying Higher-Order Neural Networks (HONN) With Limited Reservoir DataAnre Thanh HungNo ratings yet
- Production and Analysis of Pyrolysis Oil From Waste Plastic in Kolhapur CityDocument6 pagesProduction and Analysis of Pyrolysis Oil From Waste Plastic in Kolhapur CityBabatunde Olawale AfeezNo ratings yet
- Post Utme Screening For 2017/2018 Admissions Exercise: University of MaiduguriDocument1 pagePost Utme Screening For 2017/2018 Admissions Exercise: University of MaiduguriDauda BabaNo ratings yet
- High Throughput Screening of Clopidogrel Resistance Using MicroflDocument185 pagesHigh Throughput Screening of Clopidogrel Resistance Using MicroflDauda BabaNo ratings yet
- Academic Calender 2016 2017Document13 pagesAcademic Calender 2016 2017Dauda BabaNo ratings yet
- BAU Agricultural Admission List 2016/2017Document69 pagesBAU Agricultural Admission List 2016/2017Dauda BabaNo ratings yet
- Experimental Investigation of Two-Phase GAS-Liquid Slug Flow Inclined PipeDocument13 pagesExperimental Investigation of Two-Phase GAS-Liquid Slug Flow Inclined PipeDauda BabaNo ratings yet
- Comparisons of Oil Production Predicting Models: Yishen Chen, Xianfeng Ding, Haohan Liu, Yongqin YanDocument5 pagesComparisons of Oil Production Predicting Models: Yishen Chen, Xianfeng Ding, Haohan Liu, Yongqin YanDauda BabaNo ratings yet
- Development of An Adaptive Surrogate Model For Production OptimizationDocument25 pagesDevelopment of An Adaptive Surrogate Model For Production OptimizationDauda BabaNo ratings yet
- The Application of Artificial Neural Networks For The Prediction of Oil Production Flow RateDocument12 pagesThe Application of Artificial Neural Networks For The Prediction of Oil Production Flow RateDauda BabaNo ratings yet
- BAU Agricultural Admission List 2016/2017Document69 pagesBAU Agricultural Admission List 2016/2017Dauda BabaNo ratings yet
- Guidelines For Referencing Electronic SourcesDocument10 pagesGuidelines For Referencing Electronic SourcesDauda BabaNo ratings yet
- CBSE Class 7 Science - Changes & ReactionsDocument1 pageCBSE Class 7 Science - Changes & Reactionsreshma khemchandaniNo ratings yet
- Orca Share Media1679932480278 7046147521763878353Document13 pagesOrca Share Media1679932480278 7046147521763878353Endelyn Salva SajorgaNo ratings yet
- TPL Call No:-HRRL/CDU-VDU/TR-JOHN ZINK/2959 Dated 18.11.2022Document1 pageTPL Call No:-HRRL/CDU-VDU/TR-JOHN ZINK/2959 Dated 18.11.2022PRASHANTNo ratings yet
- Physical Vs Chemical Change AnswersDocument3 pagesPhysical Vs Chemical Change AnswersLaili LeliNo ratings yet
- Securitex Flammable Gas Detector ND-104N Installation GuideDocument1 pageSecuritex Flammable Gas Detector ND-104N Installation GuideMad HouseNo ratings yet
- Electrolysers - AEC, AEM, PEM and SOE For Hydrogen (And Syngas) Production - 2021 sbh4 GMBHDocument1 pageElectrolysers - AEC, AEM, PEM and SOE For Hydrogen (And Syngas) Production - 2021 sbh4 GMBHWei TengNo ratings yet
- Energy Changes PresentationDocument37 pagesEnergy Changes PresentationSadia KashifNo ratings yet
- Suggesion CRE 2022Document14 pagesSuggesion CRE 2022Soumyodeep ChowdhuryNo ratings yet
- APIChem Featured Products PDFDocument5 pagesAPIChem Featured Products PDFTezozómocNo ratings yet
- CHE 254 1 Homework 14 - Chapter 4Document2 pagesCHE 254 1 Homework 14 - Chapter 4tsbertalanNo ratings yet
- Libroflng2014 PDFDocument2 pagesLibroflng2014 PDFmchavez_579229No ratings yet
- Company ProfileDocument21 pagesCompany ProfileAbdul BasitNo ratings yet
- U Burner Boom: Shanghai Sunry Petroleum Equipment Co., LTDDocument2 pagesU Burner Boom: Shanghai Sunry Petroleum Equipment Co., LTDIndra JumenaNo ratings yet
- Assignment 1Document4 pagesAssignment 1Austin Phua Yun HockNo ratings yet
- Operating Limits of Steels in Hydrogen Service - From API RP 941Document1 pageOperating Limits of Steels in Hydrogen Service - From API RP 941Ajmi HmidaNo ratings yet
- Prop Gas Natural PDFDocument88 pagesProp Gas Natural PDFDaniel Pastor OrtíNo ratings yet
- Natural Gas and Crude Oil Sources for Petrochemical IntermediatesDocument45 pagesNatural Gas and Crude Oil Sources for Petrochemical IntermediatesGalal Eldien GalalNo ratings yet
- (Grupo 3) Wang-2021-Ligandcontrolled-tunable-coppercataDocument8 pages(Grupo 3) Wang-2021-Ligandcontrolled-tunable-coppercataVANESSA RODRIGUEZ CEBALLOSNo ratings yet
- ReliancepxDocument42 pagesReliancepxAkshata DalviNo ratings yet
- Catalysts: An Overview of The Photocatalytic Water Splitting Over Suspended ParticlesDocument25 pagesCatalysts: An Overview of The Photocatalytic Water Splitting Over Suspended ParticlescarloarchivioNo ratings yet
- Vapor Pressures and Relative Volatility of n-Pentane and n-HexaneDocument5 pagesVapor Pressures and Relative Volatility of n-Pentane and n-Hexaneaypdfiq2No ratings yet
- What Is LNGDocument4 pagesWhat Is LNGMeghali BorleNo ratings yet
- Seminar IncinerationDocument24 pagesSeminar IncinerationSaleem SalluNo ratings yet
- Kesetimbangan Uap CairDocument37 pagesKesetimbangan Uap Cairmanarul91No ratings yet
- BFF Physics and Chemistry PDFDocument13 pagesBFF Physics and Chemistry PDFtekebaNo ratings yet