Professional Documents
Culture Documents
Neurophysiologic Basis of EEG: Piotr Olejniczak
Uploaded by
ioanviOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Neurophysiologic Basis of EEG: Piotr Olejniczak
Uploaded by
ioanviCopyright:
Available Formats
ORIGINAL ARTICLES
Neurophysiologic Basis of EEG
Piotr Olejniczak
Summary: This review article introduces the reader to the very basics of electroencephalography (EEG). It tries to explain in simple terms the physiologic principles of EEG generation and organization at the cellular, cortical and subcortical levels. It also introduces the basic EEG terminology (see the key words). Key Words: EEG, Postsynaptic potentials, Volume conduction, Propagation, Synchronization, Desynchronization. (J Clin Neurophysiol 2006;23: 186189)
EEG DEFINITION What is EEG?
Electroencephalography is a graphic representation of the difference in voltage between two different cerebral locations plotted over time. The scalp EEG signal generated by cerebral neurons is modied by electrical conductive properties of the tissues between the electrical source and the recording electrode on the scalp, conductive properties of the electrode itself, as well as the orientation of the cortical generator to the recording electrode (Fig. 1).
VOLUME CONDUCTION
The EEG can be obtained because of the process of current ow through the tissues between the electrical generator and the recording electrode, which is called volume conduction. EEG provides a two-dimensional projection of three-dimensional reality, which means that theoretically it is impossible to determine the location of the EEG generator based on scalp-recorded EEG information alone. This is referred to as the inverse problem.
tinuum. Only synaptic activity readily fullls those criteria and is most signicant source of EEG potentials. Each synapse acts like a battery driving current in a small loop. Both excitatory postsynaptic potentials (EPSPs) and inhibitory postsynaptic potentials (IPSPs) contribute to the synaptic activity recorded as EEG (Fig. 2). The summation of extracellular currents is slow enough to be able to generate EEG potentials. The current owing across the external resistance of the cortex sums with the loop currents of the neighboring neurons to constitute a local mean eld (Fig. 3). Viewed from outside the cells, membrane areas where current ows in or out of the cells are called respectively sinks and sources. Excitatory currents, involving Na or Ca2 ions, ow inward toward an excitatory synapse and outward away from it. The outward current is referred as a passive return current (from intracellular to extracellular space). Inhibitory loop currents, involving Cl and K ions, ow in the opposite direction. Scalp electrodes record potential differences that are caused by postsynaptic potentials in the cell membrane of cortical neurons. The closed loops of the lighter dashed lines represent the summation of extracellular currents produced by the postsynaptic potentials; the open segments of heavier dashed lines connect all points having the same voltage level. The two scalp electrodes are at different voltage levels and record this difference (Fig. 4).
NONSYNAPTIC CONTRIBUTIONS TO EEG
The nonsynaptic activity is a less signicant source of extracellular current ow that produces EEG potentials. Intrinsic neuronal activity such as fast action potentials usually lasts too short to affect EEG. Nonsynaptic intercellular interactions may potentially contribute to EEG (Buzsaki, Traub and Pedley, 2003)
SYNAPTIC SOURCES OF EEG
To visualize and monitor minute (in the microvolt range) cerebral electrical activity, it must be of sufcient duration and sustained strength. To put it facetiously, one has to nd a platform on which both the examiner and the examined brain nd themselves in the same time-space con-
INTRINSIC NEURONAL SOURCES OF EEG
Short-lasting ( 2 s) high-amplitude individual fast (Na ) action potentials do not contribute to scalp recorded potentials except during synchronous events both physiologic such as sleep transients and pathologic such as epileptic activity. Calcium-mediated action potentials (calcium spikes) are voltage generated and occur synchronously with dendritic EPSPs. They can contribute to the creation of the dendritic eld sinks, especially during epileptiform activity
Epilepsy Center of Excellence at the LSU Health Sciences Center in New Orleans, Louisiana, U.S.A. Paper presented on 12-02-2005 at the course EEG: The Basics during the Joint Meeting of the American Epilepsy Society and the American Clinical Neurophysiology Society in Washington, D.C. Address correspondence and reprint requests to Dr. Piotr Olejniczak, Louisiana State University Healthcare Network, 5329 Didesse Street, Baton Rouge, LA 70815, U.S.A.; e-mail: polejn@lsuhsc.edu. Copyright 2006 by the American Clinical Neurophysiology Society ISSN: 0736-0258/06/2303-0186
INTRINSIC SPIKE AFTERHYPERPOLARIZATION
Intrinsic spike afterhyperpolarization (AHP) following dendritic Ca2 spikes results in suppression of fast spikes and
186
Journal of Clinical Neurophysiology Volume 23, Number 3, June 2006
Journal of Clinical Neurophysiology Volume 23, Number 3, June 2006
Neurophysiologic Basis of EEG
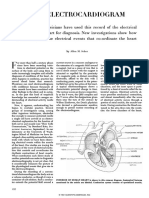
FIGURE 1. Simultaneous intracranial and scalp EEG recording of a temporal lobe seizure. The channels in the upper third of the picture represent intracranial contacts, whereas the ones in the lower part represent the scalp contacts. The intracranial recording appears to be generally more regular and of higher amplitude then the scalp one, which appears to be attenuated (from Ebersole, 2003)
FIGURE 2. Generation of extracellular voltage fields from graded synaptic activity (from Martin, 1991). Relationship between polarity of surface potentials and site of dendritic postsynaptic potentials.
hyperpolarization of the membrane caused by activation of the Ca2 -mediated K conductance. These AHPs are comparable in amplitude and duration to the synaptic events, and as such, may contribute to extracellularly recorded EEG potentials. An example of AHPs may be the generation of delta waves in sleep. In the awake brain, subcortical neurotransmitters such as acetylcholine, catecholamines and histamine reduce the calcium-mediated potassium conductance, blocking the AHP-related delta waves.
cium inux into the neurons. Postictally increased K levels produce propagating waves across the astrocytes manifested by slowly spreading sustained potentials. Astrocytes release more K , that way depolarizing the neurons and blocking the afterdischarge with resulting postictal depression.
CORTICAL GENERATORS OF EEG
The main sources of EEG derive from cerebral cortex and form three-dimensional potential elds, which can be recorded as projected two-dimensional elds in the function of voltage versus time. It takes a combined synchronous electrical activity of approximately 108 neurons in a cortical area of minimum 6 cm2 to create visible EEG. The area of cortex required for the generation of interictal spikes may be as large as 20 cm2. As mentioned earlier, the principal generators of EEG elds measured on the surface of the brain or at the scalp are graded synaptic potentials; i.e., EPSPs and IPSPs of the pyramidal neurons. At the synaptic site of an EPSP there is an active current sink (extracellular negative eld). Positive ions migrate to the cell and depolarize the membrane. At the distal part of the cell (body and distal dendrites) a passive current source out of the cell is associated with extracellular positive eld. EEG elds are primarily generated by the large, vertically oriented pyramidal neurons located in cortical layers III, V, and VI. Because of the attenuating properties of the skull, spatial (i.e., tridimensional) summation of cortical activity is critical for producing a voltage eld recordable from the scalp.
NONSYNAPTIC CELLULAR INTERACTIONS
Evoked ephaptic effects may change transmembrane potentials creating extracellular current loops that can recruit neurons to re even with insufcient activation by synaptic inputs. Ultrafast cortical rhythms result from short-lived interactions between interneurons and pyramidal cells that may produce a short-lived oscillatory eld potential (ripple) in both hippocampus and neocortex. Although not recorded by standard EEG equipment, they may be diagnostically significant harbingers of seizure onset and offset.
NEURONGLIA INTERACTIONS
The astrocytes are connected by gap junctions that allow spread of current and diffusion of molecular transport. This coupling allows spreading Ca2 waves triggering calCopyright 2006 by the American Clinical Neurophysiology Society
187
P. Olejniczak
Journal of Clinical Neurophysiology Volume 23, Number 3, June 2006
FIGURE 3. Schematic of a brain cross section, illustrating four representative cortical EEG sources (from Ebersole, 2003). Sources 2 and 3 produce radial fields, so the negative, so the negative voltage maximum is directly above them. Sources 1 and 4 produce tangential fields and both negative and positive voltage maxima are displaced to either side.
RELATIONSHIP BETWEEN POLARITY OF SURFACE POTENTIALS AND SITE OF DENDRITIC POSTSYNAPTIC POTENTIALS
Source area is maximal for a given electrode when the orientation of the active cortical region is face-on. This is the case when the voltage eld is radial and electrode right above the source. A radial source produces eld maximal directly above it, and another one with opposite polarity on the opposite side of the head. For a supercial source, the scalp maximum nearest it is signicantly greater than the one on the opposite side of the head. Single-voltage eld maximum cannot be used to dene the location and/or orientation of a cortical EEG generator. Except for a purely radial source, the EEG eld maxima are displaced from a position directly above it. The location of both negative and positive eld maxima and their relative strengths must be taken into consideration.
FIGURE 4. Relationship between EEG slow waves and thalamic intracellular potentials (from Steriade et al., 1993).
PROPAGATION OF CORTICAL ACTIVATION
With propagation of electrical activity into adjacent cortical regions, the geometry of the source changes. New location and new orientation of the source take place resulting in a different voltage eld. Movement of scalp eld maxima over tens of milliseconds suggests propagation of source activity.
SUBCORTICAL SYNCHRONIZATION OF EEG
The dorsal thalamus is considered as the chief subcortical EEG rhythm generator synchronizing populations of neocortical neurons as voltage generators. In normal conditions both thalamic nuclei and cortical regions interact to
produce the synchrony of cortical postsynaptic potentials (PSPs) during wakefulness and sleep. The facultative pacemaker theory assumes that thalamocortical relay neurons send bers to the cortex as well as give off branches that turn back and end on thalamic inhibitory interneurons (biofeedback servomechanism). Nucleus reticularis hypothesis attributes the pacemaker properties to the nucleus reticularis thalami, whose cells release the inhibitory neurotransmitter GABA in rhythmic bursts of depolarizations directed to the neurons of the dorsal thalamus and rostral brainstem. Alpha-frequency rhythms are generated in multiple cortical areas in addition to the occipitally dominant alpha rhythm. Local cortical connections seem to be more important in generating most alpha-frequency rhythms, although thalamocortical discharges may have limited inuence on posterior dominant alpha rhythm. Fast waking rhythms such as in beta- and gammafrequency bands are produced by cortical generators. Pontine cholinergic input to the thalamus inhibits the generators producing sleep transients such as sleep spindles and highamplitude delta waves. Sleep spindles appear to be a thalamocortical phenomenon. GABAergic neurons of the nucleus reticularis thalami discharge in rhythmic cycles. They project to thalamocortical relay neurons, which in turn project to widespread cortical neurons. The relay neurons discharge after GABA-release related inhibition subsides. That subsequently results in synchronized EPSPs in the cortex, which become visible by EEG.
Copyright 2006 by the American Clinical Neurophysiology Society
188
Journal of Clinical Neurophysiology Volume 23, Number 3, June 2006
Neurophysiologic Basis of EEG
Unlike sleep spindles, which require synaptic activities to establish the rhythmic oscillation, delta oscillation is an intrinsic rhythm that depends on potassium uxes at voltagedependent ion channels of cortical and thalamic neurons.
occur in the setting of widespread injury to the ascending neuronal systems that would otherwise produce arousal and desynchronization.
SUBCORTICAL EEG DESYNCHRONIZATION
Desynchronization of the EEG is the interruption of its rhythmical activity. It occurs with activation of ascending cholinergic projections of the basal forebrain and brainstem and projections from the raphe nuclei and locus ceruleus. Rhythmical activity is interrupted by both direct effects on cortical neurons and indirectly on thalamic neurons. At the cellular level, desynchronization is accompanied by a transition from a burst ring pattern to more continuous or single spike pattern. Desynchronization is enhanced by behavioral arousal and suppressed by non-REM sleep. Certain abnormal synchronous patterns, such as alpha coma,
REFERENCES
Buzsaki G, Traub RD, Pedley TA. The cellular basis of EEG activity. In: Ebersole JS, Pedley TA, eds. Current practice of clinical electroencephalography. 3rd ed. Philadelphia: Lippincott Williams and Wilkins, 2003:111. Ebersole JS. Cortical generators and EEG voltage elds. In: Ebersole JS, Pedley TA, eds. Current practice of clinical electroencephalography. 3rd ed. Philadelphia: Lippincott Williams and Wilkins, 2003:1231. Fisch BJ. Fisch and Spehlmanns EEG primer. Amsterdam: Elsevier, 1999. Martin JH. The collective electrical behavior of cortical neurons: the electroencephalogram and the mechanisms of epilepsy. In: Kandel ER, Schwartz JH, Jessel TM, eds. Principles of neural science. Norwalk: Appleton and Lange, 1991:77791. Steriade M, McCormick DA, Sejnowski T. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:67985.
Copyright 2006 by the American Clinical Neurophysiology Society
189
You might also like
- 5 EEG-historiaDocument21 pages5 EEG-historiaRodrigo A.S.No ratings yet
- Doidera EEGDocument17 pagesDoidera EEGLuís PachecoNo ratings yet
- Electroencephalography (Eeg) : Atarzyna Linowska Iotr Urka Warsaw University Warszawa, PolandDocument15 pagesElectroencephalography (Eeg) : Atarzyna Linowska Iotr Urka Warsaw University Warszawa, PolandNicholas DiazNo ratings yet
- Fundamental of EEG MeasurementDocument12 pagesFundamental of EEG MeasurementRyna Aulia Falamy100% (1)
- Review of Clinical EEGDocument200 pagesReview of Clinical EEGtuanamg66100% (9)
- The Origin of Extracellular Fields and Currents PDFDocument14 pagesThe Origin of Extracellular Fields and Currents PDFbaghlolNo ratings yet
- NRN 3241Document14 pagesNRN 3241hussin elrashidyNo ratings yet
- Kuperberg - Electroencephalography. Capitulo 6 PersDocument12 pagesKuperberg - Electroencephalography. Capitulo 6 PersAlejandra GaonaNo ratings yet
- mfd2011 Basis MEEGDocument49 pagesmfd2011 Basis MEEGMichael HabibNo ratings yet
- EEG and Epilepsy Monitoring.10Document25 pagesEEG and Epilepsy Monitoring.10mhd.mamdohNo ratings yet
- EEG Measurement Fundamentals ReviewDocument11 pagesEEG Measurement Fundamentals Reviewfiziomar8100% (2)
- Perspectives on Evidence for an Electrical Analog System Controlling Brain FunctionDocument18 pagesPerspectives on Evidence for an Electrical Analog System Controlling Brain FunctionordoNo ratings yet
- Intervention: Electrical Scalp Neurons BrainDocument25 pagesIntervention: Electrical Scalp Neurons BrainnikitatayaNo ratings yet
- Electricity in The BodyDocument6 pagesElectricity in The BodyAnnika Erickson100% (1)
- Evoked Potentials: Principles and TechniquesDocument5 pagesEvoked Potentials: Principles and TechniquesLulu SupergirlNo ratings yet
- Bioelectric Signals and ElectrodesDocument20 pagesBioelectric Signals and Electrodesنذير الشرعبيNo ratings yet
- EEG Anubhav 071221Document58 pagesEEG Anubhav 071221Harsh TanwarNo ratings yet
- Electroencephalography: Electroencephalography (EEG) Is The Recording ofDocument1 pageElectroencephalography: Electroencephalography (EEG) Is The Recording ofPirate MayurNo ratings yet
- Origin and Technical Aspects of The EEGDocument37 pagesOrigin and Technical Aspects of The EEGbaba ababNo ratings yet
- Husos de SueñoDocument37 pagesHusos de SueñoYair FloresNo ratings yet
- Electroencephalography: Source of EEG ActivityDocument16 pagesElectroencephalography: Source of EEG ActivityEmonNo ratings yet
- 10 - Chapter - 01 Seizure DetectionDocument21 pages10 - Chapter - 01 Seizure DetectionSuguna NanthiniNo ratings yet
- Powerlab: Physiology of The Nerve: KeywordsDocument7 pagesPowerlab: Physiology of The Nerve: KeywordsCJ RanesesNo ratings yet
- Bio SignalsDocument36 pagesBio SignalsVivek NamaniNo ratings yet
- The Design of A Cuff Electrode For Long Term Neural Signal Recordingwith Selective Recording Capability To Rehabilitate People With NerveDamageDocument10 pagesThe Design of A Cuff Electrode For Long Term Neural Signal Recordingwith Selective Recording Capability To Rehabilitate People With NerveDamageInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Cortical Dipole and Clinical SignificanceDocument15 pagesCortical Dipole and Clinical SignificanceManamitaNo ratings yet
- Electrical Resonance With Voltage-Gated Ion ChannelsDocument8 pagesElectrical Resonance With Voltage-Gated Ion ChannelsNusrat JahanNo ratings yet
- Tinku Biswas, Swarup Sarkar, Akash Ku. Bhoi, Surajit BagchiDocument7 pagesTinku Biswas, Swarup Sarkar, Akash Ku. Bhoi, Surajit BagchiInternational Journal of computational Engineering research (IJCER)No ratings yet
- Clinical Neurophysiology and Electroencephalography: Jong Woo Lee, MD, PHD, and Shahram Khoshbin, MDDocument9 pagesClinical Neurophysiology and Electroencephalography: Jong Woo Lee, MD, PHD, and Shahram Khoshbin, MDEmilio SanchezNo ratings yet
- EEGDocument16 pagesEEGadibaronessNo ratings yet
- Eggermont 2019 ABRDocument15 pagesEggermont 2019 ABRSujeet PathakNo ratings yet
- CES Article - Jan 26-2015Document14 pagesCES Article - Jan 26-2015LidaConstanzaMendezNo ratings yet
- 1985 DendriticspinesDocument4 pages1985 Dendriticspinesangelamm1No ratings yet
- 1 s2.0 S1053811923003300 MainDocument11 pages1 s2.0 S1053811923003300 Mainmawara khanNo ratings yet
- Project Report On EEG MachineDocument26 pagesProject Report On EEG Machineneetubansal60% (5)
- Systemic Study of Devices Processing Electromagnetic Waves Application To Brain Waves and EmotionsDocument5 pagesSystemic Study of Devices Processing Electromagnetic Waves Application To Brain Waves and EmotionsInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Reference-Free Quantification of EEG Spectra: Combining Current Source Density (CSD) and Frequency Principal Components Analysis (fPCA)Document21 pagesReference-Free Quantification of EEG Spectra: Combining Current Source Density (CSD) and Frequency Principal Components Analysis (fPCA)Raynald SumampouwNo ratings yet
- Grupo 1 Practica 14Document12 pagesGrupo 1 Practica 14Sadit Chauca VelaNo ratings yet
- Nervous System Communication MethodsDocument9 pagesNervous System Communication MethodsSherida GibbsNo ratings yet
- Electroencephalography (Eeg)Document31 pagesElectroencephalography (Eeg)Nidhin Thomas100% (3)
- CH 04 DDocument34 pagesCH 04 DVijayRajNo ratings yet
- BM Unit-IIIDocument12 pagesBM Unit-IIIHp KNo ratings yet
- Electroencephalography: Source of EEG ActivityDocument16 pagesElectroencephalography: Source of EEG ActivityJames LevyNo ratings yet
- Simulate EEG SignalDocument7 pagesSimulate EEG SignaldipakNo ratings yet
- Excitability of Neurons and Glial CellsDocument9 pagesExcitability of Neurons and Glial CellsEusebio ChaconNo ratings yet
- Duffy The - BEAM - Method - For - Neurophysiological - DDocument16 pagesDuffy The - BEAM - Method - For - Neurophysiological - DMaria Jose FigueroaNo ratings yet
- Biomedical applications of electrical potentialsDocument3 pagesBiomedical applications of electrical potentialstanti lutfiaNo ratings yet
- Morales1978 AtoniaDocument7 pagesMorales1978 AtoniasujNo ratings yet
- The Electrocardiogram: Allen M. ScherDocument12 pagesThe Electrocardiogram: Allen M. ScherrkukgNo ratings yet
- Energetic HeartDocument22 pagesEnergetic HeartkatburnerNo ratings yet
- FinalDocument39 pagesFinallinhptt14012000No ratings yet
- Emg Conceptos - Nerve Conduction StudiesDocument23 pagesEmg Conceptos - Nerve Conduction StudiesDeborah SalinasNo ratings yet
- EPSP TutorialDocument5 pagesEPSP TutorialHanz OceNo ratings yet
- Medical Devices Which Use ElectrodesDocument2 pagesMedical Devices Which Use ElectrodesAnonymous LCYYwcNo ratings yet
- EEG Report: A Guide to ElectroencephalographyDocument11 pagesEEG Report: A Guide to ElectroencephalographyLuiejen GasconNo ratings yet
- Paper 1Document3 pagesPaper 1MALEPATI ASHRITHANo ratings yet
- EKG/ECG Interpretation Made Easy: A Practical Approach to Passing the ECG/EKG Portion of NCLEXFrom EverandEKG/ECG Interpretation Made Easy: A Practical Approach to Passing the ECG/EKG Portion of NCLEXRating: 5 out of 5 stars5/5 (2)
- Neurofeedback - The Neurofeedback Book for Patients and Therapists : A Symphony of the MindFrom EverandNeurofeedback - The Neurofeedback Book for Patients and Therapists : A Symphony of the MindNo ratings yet
- Etonitazene - An Opioid Selective For The Mu Receptor Types - Moolten MS, Fishman JB, Chen JC, Carlson KR - Life Sci 52 (1993) PL199-PL203Document5 pagesEtonitazene - An Opioid Selective For The Mu Receptor Types - Moolten MS, Fishman JB, Chen JC, Carlson KR - Life Sci 52 (1993) PL199-PL203muopioidreceptorNo ratings yet
- Fuad Lechin, Bertha Van Der Dijs, Gerardo Herna Ndez-Adria NDocument21 pagesFuad Lechin, Bertha Van Der Dijs, Gerardo Herna Ndez-Adria NMaria Lucrecia CrespoNo ratings yet
- Ch03 Questions Answer KeyDocument18 pagesCh03 Questions Answer KeyMichaelNo ratings yet
- Action PotentialDocument12 pagesAction PotentialArooj FatimaNo ratings yet
- Neurons and Its FunctionDocument4 pagesNeurons and Its FunctionKristol MyersNo ratings yet
- Neurotransmission Notes 3Document4 pagesNeurotransmission Notes 3api-188978784No ratings yet
- L 55 Introduction To Hormones and Hormone Receptors (CH 75)Document15 pagesL 55 Introduction To Hormones and Hormone Receptors (CH 75)Tayai LengimaNo ratings yet
- Jeopardy Review - AP Bio Cell Communication PDFDocument34 pagesJeopardy Review - AP Bio Cell Communication PDFJhane FigueroaNo ratings yet
- Chapter 2 - Receptor TheoryDocument6 pagesChapter 2 - Receptor TheoryChantal CarnesNo ratings yet
- Module 3 - Neural PhsyiologyDocument49 pagesModule 3 - Neural Phsyiologybuy sellNo ratings yet
- Gustatory (Taste) Receptors: Dr. Rusdi, M.BiomedDocument16 pagesGustatory (Taste) Receptors: Dr. Rusdi, M.BiomedNahla FaridaNo ratings yet
- Human Physiology Assignment 01Document9 pagesHuman Physiology Assignment 01Evaristo MK VincentNo ratings yet
- Muscle RelaxantDocument29 pagesMuscle RelaxantAri Puji AstutiNo ratings yet
- Cannabinoid-Based Drugs As Anti-Inflammatory TherapeuticsDocument12 pagesCannabinoid-Based Drugs As Anti-Inflammatory TherapeuticsAldo BustamanteNo ratings yet
- Ion Channels of Excitable Membranes: Bertil HilleDocument11 pagesIon Channels of Excitable Membranes: Bertil HilleIgnacio Martinez Garcia100% (1)
- !03 Glutamate ReceptorDocument61 pages!03 Glutamate ReceptorNahla FaridaNo ratings yet
- Neuron System QuestionDocument15 pagesNeuron System QuestionShela Huang100% (1)
- Text 4Document62 pagesText 4geri delaneyNo ratings yet
- Evolution of Cell SignalingDocument3 pagesEvolution of Cell SignalingkatakanNo ratings yet
- In-Class Quiz Review - NeurophysiologyDocument7 pagesIn-Class Quiz Review - NeurophysiologyWaqas GillNo ratings yet
- Advanced Cell BiologyDocument14 pagesAdvanced Cell BiologyUmair AneesNo ratings yet
- Memory Eric KandelDocument6 pagesMemory Eric KandelJorge ChacónNo ratings yet
- 2&3 - Action Potential and Resting MembraneDocument28 pages2&3 - Action Potential and Resting MembraneTejesswinNo ratings yet
- Alpha7 Nicotinic Acetylcholine Receptor Is A Target in Pharmacology and ToxicologyDocument20 pagesAlpha7 Nicotinic Acetylcholine Receptor Is A Target in Pharmacology and ToxicologyJean Pierre Chastre LuzaNo ratings yet
- Neuromuscular Blocking AgentsDocument18 pagesNeuromuscular Blocking AgentsLalibah AntartikaNo ratings yet
- β2 Agonists Mechanism of Action and Drug ExamplesDocument13 pagesβ2 Agonists Mechanism of Action and Drug ExamplesMohamed IsmailNo ratings yet
- PA2 Value: A Pharmacological Scale to Express Drug AntagonismDocument22 pagesPA2 Value: A Pharmacological Scale to Express Drug AntagonismSaifali SurtiNo ratings yet
- Daily Test Class 11 - 4thDocument5 pagesDaily Test Class 11 - 4thDebby GraciaNo ratings yet
- Print Sign Your Name Sign Do Not: Exam 3 - Zoology 250Document7 pagesPrint Sign Your Name Sign Do Not: Exam 3 - Zoology 250HUAWEI HUAWEINo ratings yet
- Chapter 12 PDFDocument14 pagesChapter 12 PDFJeanPaule Joumaa100% (1)