Professional Documents
Culture Documents
Isolation, Hydrolysis & Characterization of Proteins
Uploaded by
Reysa Gabrielle PileOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Isolation, Hydrolysis & Characterization of Proteins
Uploaded by
Reysa Gabrielle PileCopyright:
Available Formats
Isolation, Hydrolysis and Characterization of Protein Objectives: -to isolate proteins (gluten, casein, albumin, and myoglobin) -to
hydrolyze proteins -to perform color reactions to intact protein and hydrolysate -to determine the concentration of protein using Bradford assay Proteins-polymers of amino acids linked head to tail, from carboxyl group to amino group, through formation of covalent peptide bonds Amino acids -building blocks of proteins -carboxylic acid group -amino group -side group R gives unique characteristics R side chain | H2H C-COOH | H Classification of Protein I. Biological function 1. 2. 3. 4. 5. 6. 7. 8. II. Structure- cellulose, keratin, collagen Catalysis- enzymes Movement/Contraction- myosin, actin Transport- hemoglobin, transferring Hormones- insulin Protection- antibodies, fibrinogen Storage- casein, ovalbumin RegulationThree-dimensional shape or conformation
1. fibrous- keratin, collagen, elastin Are organized into linear or sheetlike structures with a regular repeating folding pattern 2. globular- casein, albumin, hormones Are folded into compact, nearly spherical, globular conformation III. Composition
1. Simple- contains only amino acids and their derivatives
2. Conjugated- apart from amino acids, it may contain a carbohydrate-, lipid-, or phosphomoiety or groups Conjugated proteins Glycoproteins are proteins containing carbohydrate groups Lipoproteins are proteins that are associated with lipid molecules Nucleoproteins are proteins joined with nucleic acid Phosphoproteins contain phosphate groups Metalloproteins are protein-metal complexes Hemaproteins contain heme Flavoproteins contain riboflavin Hierarchy of Protein Structure 1. Primary structure- the sequence of amino acids 2. Secondary structure- the regular, repeating folding pattern 3. Tertiary structure- the way that segments of the protein fold in three dimensions; over all folding of domains 4. Quaternary structures- the interaction between different polypeptide chains to produce an oligometric structure Isolation of Proteins 1. Casein- isoelectric precipitation a phosphoprotein which has phosphate groups attached to some amino side chains exists in milk as calcium caseinate calcium caseinate has its isoelectric point at 4.6 Ca2Caseinate + 2HCl Casein + CaCl2 2. Albumin denaturation and coagulation by heat Are globular proteins that are soluble in water and in dilute salt solutions 3. Gluten- difference in solubility A protein found in wheat, rye, and barley Gives bread its structure, texture, and elasticity It is a composite of a prolamin and a glutelin which exist, conjoined with starch 4. Myoglobin- salt-induced precipitation ising (NH4)2 SO4 Protein Hyrdolysis Break down of peptide bonds Requires acid or base, water, and heat Gives smaller peptides and amino acids Similar to digestion of proteins using enzymes Occurs in cells to provide amino acids to synthesize other proteins and tissues Types of Hydrolysis Acidic
Basic Enzymatic
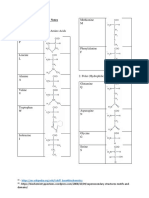
Acidic Hydrolysis Hydrolysate is neutralized with NaOH/ Ba(OH)2 Complete Hydrolysis No racemization of Amino Acids occur Tryptophan is destroyed and convented to humin Asparagine and glutamine are converted to aspartic acid and glutamic acid, respectively. Basic Hydrolysis Rxn mixture is autoclaved at 15psi for 5 hrs with Na(OH)/ Ba(OH)2 Hydrolysate is neutralized with HCl/ H2SO4 Complete hydrolysis Racemization of all AA occur Tryptophan is stable Arginine is hydrolyzed into ornithine and urea Cysteine, serine, and threonine are destroyed Enzymatic Hydrolysis Incomplete hydrolysis Exopeptidase/Endopeptidase Denaturation- Disruption of Secondary, tertiary, and quaternary protein structure by: Heat/organics-break apart H bonds and disrupt hydrophobic attractions Acids/Bases- Break H bonds between polar R groups and ionic bonds Heavy metal ions- react with S-S bonds to form solids Agitation- stretches chains until bonds break Qualitative Chemical test 1. Biuret Test Reagents: CuSO4, NaOH Relative result: violet coloration Principle involved: complexation of Cu2+ with a peptide bond 2. Ninhydrin Test Purpose: detects free alpha amino acid Positive result: blue to blue-violet color Principle involved: oxidative decarboxylation and deamination followed by condensation 3. Xanthroproteic test Reagents: conc. HNO3, conc. NaOH Purpose: test for aromatic amino acids (Y, W, and F) Positive result: yellow soln on heating; orange soln with excess NaOH
4.
5.
6.
7.
8.
9.
Principle involved: nitration of aromatic ring via electrophilic aromatic substitution Millons/Millon-Nasse test Reagents: Hg SO4 in H2SO4 Purpose: detect phenolic group in tyrosine Positive result: old rose/flesh/purple-red ppt Principle involved: complexation of nitrohydroxyphenyl derivatives with Hg3+ Hopkins-Cole test Reagents: glyoxylic adic (Mg powder, oxalic acid, acetic acid), conc. H2SO4 Purpose: specific test for indole group of tryptophan Positive result: violet interface Principle involved: acid-catalyzed condensation of two tryptophans with glyoxilic acid Sakaguchi test Purpose: specific test for guanido group of arginine Positive result: res to red-orange color Base-catalyzed condensation of napthanol with the guanido group of arginine Fohls/ Lead acetate test Reagents: Lead(II) acetate, NaOH Purpose: detects S-containing AA (methionine, cysteine) Positive result: brown or black ppt Principle involved: degradation and substitution reaction to form lead sulfide Paulys test/ Diazo reaction Reagents: NaNO2, sulfanilic acid, Na2CO3 Purpose: detects the presence of histidine and tyrosine Positive result: red color Principle involved: diazotized sulfanilic acid couples with amino phenod to form a colored azo compound in cold condition Nitroprusside test Purpose: detects the presence of SH group (cysteine) Positive result: Red ppt Principle involved: complexation
You might also like
- Biochem Proteins LabDocument3 pagesBiochem Proteins LabDaneva ReyesNo ratings yet
- 2 Water The Solvent For Biochemical ReactionsDocument68 pages2 Water The Solvent For Biochemical ReactionsSiej Go100% (1)
- Analysis of Lipids in Egg YolkDocument2 pagesAnalysis of Lipids in Egg YolkHydieNo ratings yet
- Experiment 8 Pre-Lab Comparative Reactions of Carboxylic Acid DerivativesDocument3 pagesExperiment 8 Pre-Lab Comparative Reactions of Carboxylic Acid Derivativesgian odell100% (1)
- Characteristic Reactions of Organic HalidesDocument4 pagesCharacteristic Reactions of Organic HalidesDANIEL CARLOS SALIPSIPNo ratings yet
- Analysis of LipidDocument6 pagesAnalysis of LipidanaNo ratings yet
- Week 2 Practical - Chemistry of CarbohydratesDocument7 pagesWeek 2 Practical - Chemistry of CarbohydratesPranabes Bhattacharyya100% (1)
- Pharmaceutical biochemistry lab activity on salivary digestionDocument9 pagesPharmaceutical biochemistry lab activity on salivary digestiondaven100% (1)
- Isolation of RNADocument2 pagesIsolation of RNAALLEN SERGIO DIZONNo ratings yet
- Case Study VIDocument1 pageCase Study VIShashidharan MenonNo ratings yet
- Chapter 2 Lipids Study GuideDocument10 pagesChapter 2 Lipids Study GuideJanNo ratings yet
- Composition of Living Matter ExplainedDocument3 pagesComposition of Living Matter Explainedbioinfo100% (4)
- Isolation and Qualitative Analysis of Nucleic Acids (DNA From Onion)Document3 pagesIsolation and Qualitative Analysis of Nucleic Acids (DNA From Onion)Elina Lantion100% (1)
- Sample Lab Report 5 Alain NowDocument10 pagesSample Lab Report 5 Alain NowNur SetsuNo ratings yet
- Glucose MethodologiesDocument3 pagesGlucose MethodologiesprincessNo ratings yet
- Ateneo de Zamboanga University Salivary Amylase ActivityDocument5 pagesAteneo de Zamboanga University Salivary Amylase Activity3amabelle arevaloNo ratings yet
- Carbohydrate Test Lab Report 2.2Document9 pagesCarbohydrate Test Lab Report 2.2Kryza Mariz CabreraNo ratings yet
- POstlab Biochem Experiment 4 8BDocument80 pagesPOstlab Biochem Experiment 4 8BGeline Joy D. Samillano60% (5)
- Experiment 1: Transport Across The MembraneDocument9 pagesExperiment 1: Transport Across The MembraneMerli Ann Joyce CalditoNo ratings yet
- E LabDocument3 pagesE LabAlthea ValenzuelaNo ratings yet
- POLYSACCHARIDESDocument2 pagesPOLYSACCHARIDESYholzManioNo ratings yet
- NMAT Social Science Practice Questions Set 2Document7 pagesNMAT Social Science Practice Questions Set 2thea kyla angelaNo ratings yet
- HtwoO and BufferDocument7 pagesHtwoO and BufferManila MedNo ratings yet
- ProteinsDocument8 pagesProteinsNara100% (1)
- Biochem Lab Reviewer Midterms With EditsDocument8 pagesBiochem Lab Reviewer Midterms With EditsDyosAra100% (1)
- Exercise Proteins and EnzymesDocument4 pagesExercise Proteins and EnzymesBibek GajmerNo ratings yet
- General Chemistry Laboratory Manual: Yildiz Technical University Faculty of Art & Science Chemistry DepartmentDocument36 pagesGeneral Chemistry Laboratory Manual: Yildiz Technical University Faculty of Art & Science Chemistry DepartmentLOLONo ratings yet
- Chromic Acid TestDocument2 pagesChromic Acid TestJordan MachanumNo ratings yet
- EXPERIMENT 8 Isolation of PolysaccharidesDocument3 pagesEXPERIMENT 8 Isolation of PolysaccharidesDarlene Mae GerosagaNo ratings yet
- Isolation and Characterization of CarbohydratesDocument4 pagesIsolation and Characterization of CarbohydratesEvans DionNo ratings yet
- Oral Rehydration SaltsDocument3 pagesOral Rehydration SaltsKadek Adit WiryadanaNo ratings yet
- Experiment 8 and 9 PDFDocument17 pagesExperiment 8 and 9 PDFKrizzi Dizon GarciaNo ratings yet
- Formulas for Reducing and Enlarging Pharmaceutical PreparationsDocument12 pagesFormulas for Reducing and Enlarging Pharmaceutical PreparationsDiniela CaballesNo ratings yet
- Colloids Experiment No. 2Document5 pagesColloids Experiment No. 2Chris K. Ramirez100% (1)
- Post Lab. Exs. 8-35 PhyanaDocument33 pagesPost Lab. Exs. 8-35 PhyanaChesmar Macapala100% (7)
- Experiment 2 - Laboratory Activity Sheet For Physical and Chemical Properties of ProteinsDocument5 pagesExperiment 2 - Laboratory Activity Sheet For Physical and Chemical Properties of ProteinsDelosreyes ChildrenNo ratings yet
- Experiment 9 Properties of ProteinsDocument5 pagesExperiment 9 Properties of ProteinsLara MonevaNo ratings yet
- QuestionsDocument5 pagesQuestionsTims WatsonsssNo ratings yet
- Unsaturated HydrocarbonsDocument84 pagesUnsaturated HydrocarbonsHey itsJamNo ratings yet
- Qualitative Analysis of CarbohydratesDocument17 pagesQualitative Analysis of CarbohydratessushilNo ratings yet
- Qualitative Tests For Lipids: ExperimentDocument7 pagesQualitative Tests For Lipids: ExperimentAlthea Aubrey AgbayaniNo ratings yet
- Isolation and Characterization of ProteinsDocument3 pagesIsolation and Characterization of ProteinsLor Sales0% (1)
- YB2 - EXPT2 - Apostol and Santos PDFDocument12 pagesYB2 - EXPT2 - Apostol and Santos PDFCaryll ApostolNo ratings yet
- BC34.1 E6 Isolation of GlycogenDocument7 pagesBC34.1 E6 Isolation of GlycogenGlenn Vincent Tumimbang0% (1)
- Topic 1 6 CPH PDFDocument21 pagesTopic 1 6 CPH PDFJoshua AmancioNo ratings yet
- Qualitative Test For CarbohydratesDocument4 pagesQualitative Test For CarbohydratesmegmayorNo ratings yet
- Preparation 7 Additional Post LabDocument1 pagePreparation 7 Additional Post LabKate MendozaNo ratings yet
- Experiment 3 - Laboratory Activity Chemical Tests For The Components of Nucleic AcidDocument4 pagesExperiment 3 - Laboratory Activity Chemical Tests For The Components of Nucleic AcidDelosreyes ChildrenNo ratings yet
- Carbohydrate Identification Lab ReportDocument3 pagesCarbohydrate Identification Lab ReportAbby100% (1)
- Characterization of Carbohydrates Using Thin-Layer Chromatography and Nelson's MethodDocument6 pagesCharacterization of Carbohydrates Using Thin-Layer Chromatography and Nelson's MethodPatricia Isabel Tayag60% (5)
- Activity 4Document5 pagesActivity 4Althea ValenzuelaNo ratings yet
- Experiment No. 4 GLYCOGENDocument2 pagesExperiment No. 4 GLYCOGENMissy Arabella PameNo ratings yet
- Chemlab Report2Document15 pagesChemlab Report2Czarriel F. Flores100% (1)
- Biochem Laboratory MidtermDocument15 pagesBiochem Laboratory MidtermNica DonioNo ratings yet
- Qualitative Analysis of Myoglobin and Its HydrolysateDocument7 pagesQualitative Analysis of Myoglobin and Its Hydrolysatelorenzrael26No ratings yet
- Experiment 2: Postlab Qualitative Tests For Proteins RecallDocument15 pagesExperiment 2: Postlab Qualitative Tests For Proteins RecallGrace HernandezNo ratings yet
- ProteinsDocument48 pagesProteinsSulaiman Tahsin100% (1)
- PowerPoint Presentation-3 PDFDocument53 pagesPowerPoint Presentation-3 PDFJustine Salvo EvaristoNo ratings yet
- 10 - BICH 200 ProteinDocument30 pages10 - BICH 200 ProteinDR. ANUPAMA NAGARAJNo ratings yet
- Chapter 3 - Amino Acids Peptides & ProteinsDocument43 pagesChapter 3 - Amino Acids Peptides & Proteinsdaniel3676No ratings yet
- CATALYTIC ACTIVITY OF DENATURED INVERTASEDocument3 pagesCATALYTIC ACTIVITY OF DENATURED INVERTASEReysa Gabrielle PileNo ratings yet
- FossilsDocument1 pageFossilsReysa Gabrielle PileNo ratings yet
- FeminismDocument1 pageFeminismReysa Gabrielle PileNo ratings yet
- Physics Lab ReportDocument3 pagesPhysics Lab ReportReysa Gabrielle PileNo ratings yet
- Pearson Square: Crude Protein Requirement of GoatsDocument7 pagesPearson Square: Crude Protein Requirement of GoatsRoxlee Joy AcorinNo ratings yet
- Bioquimica y Biologia Celular de Las Complicaciones de La Hiperglucemia (Diabetes Tipo II)Document28 pagesBioquimica y Biologia Celular de Las Complicaciones de La Hiperglucemia (Diabetes Tipo II)Vulcano JerezNo ratings yet
- In Silico Molecular Docking Studies of Meroditerpenoids of Stypopodium Flabelliforme Against FOXO1Document4 pagesIn Silico Molecular Docking Studies of Meroditerpenoids of Stypopodium Flabelliforme Against FOXO1Rakesh DavellaNo ratings yet
- Motor proteins use ATP to power cellular movementsDocument16 pagesMotor proteins use ATP to power cellular movementserichaasNo ratings yet
- Insulin ResistanceDocument275 pagesInsulin ResistanceHoopmen Silaen100% (7)
- Apoptosis and CancerDocument20 pagesApoptosis and CancerSajjad Ahmad0% (1)
- EcoRI - WikipediaDocument12 pagesEcoRI - WikipediaArjun ArulvelNo ratings yet
- CoagulationDocument3 pagesCoagulationHerho-nyl CesNo ratings yet
- Koagulasi DarahDocument2 pagesKoagulasi DarahSekar NugraheniNo ratings yet
- Bacillus Sp. PAMC22265 Chromosome, Complete GenomeDocument1,012 pagesBacillus Sp. PAMC22265 Chromosome, Complete GenomeltNo ratings yet
- Coagulation Made EasyDocument27 pagesCoagulation Made EasyHaris TanNo ratings yet
- Mechanism of Drug ActionDocument24 pagesMechanism of Drug ActionBandita Datta100% (1)
- Chem 145: Amino Acid Notes and PropertiesDocument12 pagesChem 145: Amino Acid Notes and PropertiesjlngnNo ratings yet
- Chapter 2 - NotesDocument22 pagesChapter 2 - NotesanusoumyaNo ratings yet
- Media 3Document313 pagesMedia 3Antonio CordobaNo ratings yet
- Review: Translational Regulation of Gene Expression During Conditions of Cell StressDocument10 pagesReview: Translational Regulation of Gene Expression During Conditions of Cell StressBayan GhanimNo ratings yet
- BM101: Key Functions and Structures of ProteinsDocument23 pagesBM101: Key Functions and Structures of Proteinshimanshu singhNo ratings yet
- Bi0505 LabDocument102 pagesBi0505 LabJatinMittalNo ratings yet
- Enzymes AnsweredDocument3 pagesEnzymes Answeredyep okNo ratings yet
- Protocols For The Analytical Characterization of Therapeutic Monoclonal Antibodies. II - Enzymatic and Chemical Sample Preparation PDFDocument11 pagesProtocols For The Analytical Characterization of Therapeutic Monoclonal Antibodies. II - Enzymatic and Chemical Sample Preparation PDFyun baiNo ratings yet
- Lembar Cek LabDocument2 pagesLembar Cek LabKarlina OktantiNo ratings yet
- Endothelium in Thrombosis JournalDocument6 pagesEndothelium in Thrombosis JournalRahmi NisaNo ratings yet
- Molecular Cell Biology Lodish 6th Edition Test BankDocument24 pagesMolecular Cell Biology Lodish 6th Edition Test BankRobertCookynwk100% (31)
- CLIC5 in PodocytesDocument15 pagesCLIC5 in PodocytesKarina ThiemeNo ratings yet
- HemostasisDocument23 pagesHemostasisSwathi BNo ratings yet
- Virology Journal: Predicting The Subcellular Localization of Viral Proteins Within A Mammalian Host CellDocument8 pagesVirology Journal: Predicting The Subcellular Localization of Viral Proteins Within A Mammalian Host CellRuy Lopez ClosedNo ratings yet
- Steroid Hormones! Mechanisms of Action!Document14 pagesSteroid Hormones! Mechanisms of Action!leh.mo9315No ratings yet
- Mus Musculus Transformation Related Protein 73 (Trp73), Transcript Variant 3, mRNADocument7 pagesMus Musculus Transformation Related Protein 73 (Trp73), Transcript Variant 3, mRNAEeeeeeeeeNo ratings yet
- 100 Concepts - MCBDocument316 pages100 Concepts - MCBNisreen SalameNo ratings yet
- RibosomesDocument12 pagesRibosomesEnika baidenNo ratings yet